GenSight Biologics announced the publication of outcomes data from 5 years’ follow-up of patients treated unilaterally with Lumevoq, the company’s investigational gene therapy for Leber hereditary optic neuropathy (LHON) due to a mutated ND4 mitochondrial gene. The data from the RESTORE study, published online by JAMA Ophthalmology in December 2024, found that patients “demonstrated a sustained bilateral improvement in BCVA and a good safety profile over 5 years after treatment.” The “persistent benefit” continues the durable effect observed at earlier time points and represents a significant addition to the body of evidence on the benefit-risk ratio of Lumevoq gene therapy in ND4 LHON patients, the company said.
According to GenSight Biologics, RESTORE is one of the largest long-term follow-up studies for a rare disease treatment, with 62 participants accepting the invitation to enroll. All participants were treated with a single intravitreal injection of Lumevoq in 1 eye and with sham injection in the other. The patients had all participated in the phase 3 trials RESCUE and REVERSE and agreed to enroll in RESTORE at the end of those studies, the company said in a press release.
“The findings on the persistence of visual improvement show the promise of using Lumevoq gene therapy to treat patients with LHON due to the MT-ND4 gene variant,” noted Patrick Yu-Wai-Man, MD, PhD, professor of ophthalmology and honorary consultant neuroophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, United Kingdom. He was the principal investigator and lead author in the RESCUE, REVERSE, and RESTORE studies. “The results are especially encouraging given the poor visual prognosis of MT-ND4 LHON, which is the most frequent and severe clinical form of this rare mitochondrial genetic disease.”
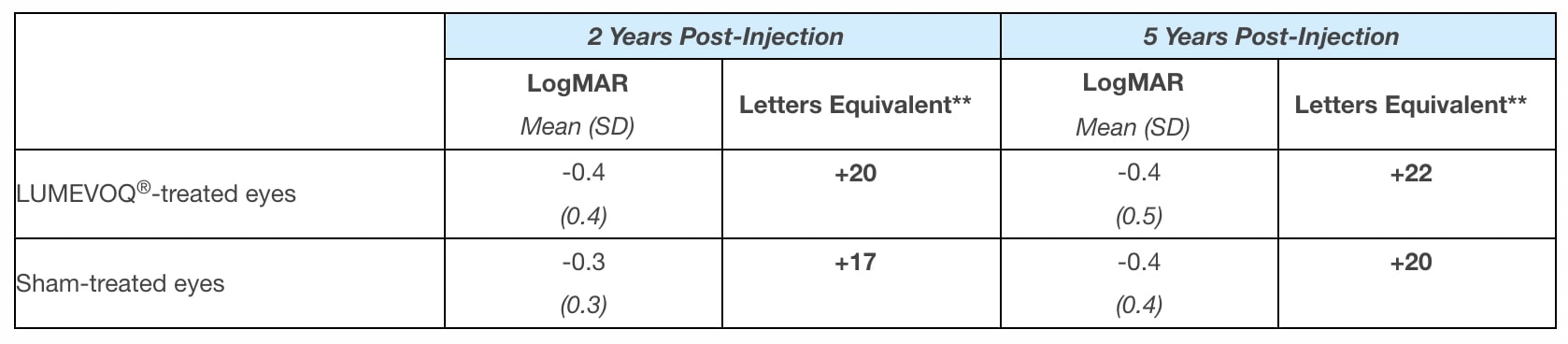
GenSight Biologics noted that when RESTORE participants enrolled in the study, 2 years after the 1-time injection, they had already experienced clinically meaningful improvement relative to the lowest point (the “nadir”) of their best-corrected visual acuity (BCVA): +20 ETDRS letters equivalent in their Lumevoq-treated eyes and +17 ETDRS letters equivalent in their sham-treated eyes. Five years after treatment, the bilateral improvement from nadir was sustained, with Lumevoq-treated eyes achieving a mean improvement against nadir of +22 letters equivalent and sham-treated eyes demonstrating a mean improvement of +20 letters equivalent.
Table 1: Change in BCVA vs. nadir* in Lumevoq long-term follow-up (RESTORE).
According to the company, responder analyses at year 5 indicate that improved BCVA was a benefit for a substantial proportion of the study participants. 66.1% of RESTORE participants achieved clinically meaningful (at least +3 lines improvement) from nadir in at least one eye, and the proportion rises to 71.0% if the criterion used is clinically relevant recovery (CRR) against nadir. (CRR corresponds to an improvement of at least 0.2 LogMAR for on-chart eyes or a movement from off-chart to on-chart.) At the end of the 5-year follow-up period, 80.6% of participants had on-chart vision (BCVA ≤ 1.6 LogMAR) in at least 1 eye.
The impact of such results on patients is demonstrated by increases in the self-reported quality of life scores at year 5 vs baseline, the company noted. Clinically significant improvement from baseline was observed in 7 of 10 subscale scores of the NEI VFQ-25 questionnaire used to assess quality of life. The composite score showed a clinically meaningful gain of 7 points from baseline.
Safety findings at 5 years after injection were consistent with previous readouts, which concluded that Lumevoq is well tolerated. The systemic safety was excellent and most ocular events were mild, none were severe or serious, and none led to study discontinuation, the company reported.