Stargardt disease (STGD) is the most common inherited macular dystrophy, with an estimated prevalence of 1 in 10,000 individuals and a clinical onset that can range from childhood to adulthood.1,2 The condition is caused by biallelic mutations in the ABCA4 gene, which disrupt the clearance of all-trans-retinal derivatives from photoreceptor outer segments. This defect leads to the accumulation of toxic bisretinoids, such as A2E, within the retinal pigment epithelium (RPE). The buildup of lipofuscin in the RPE drives cellular dysfunction and progressive photoreceptor degeneration.3 The resulting vision loss can be substantial and has a profound impact on patients’ quality of life, underscoring the urgent need for effective treatments.4
Currently, there are no approved therapies for STGD; however, multiple promising strategies are under active investigation. This article reviews recent advances in the therapeutic pipeline for STGD.
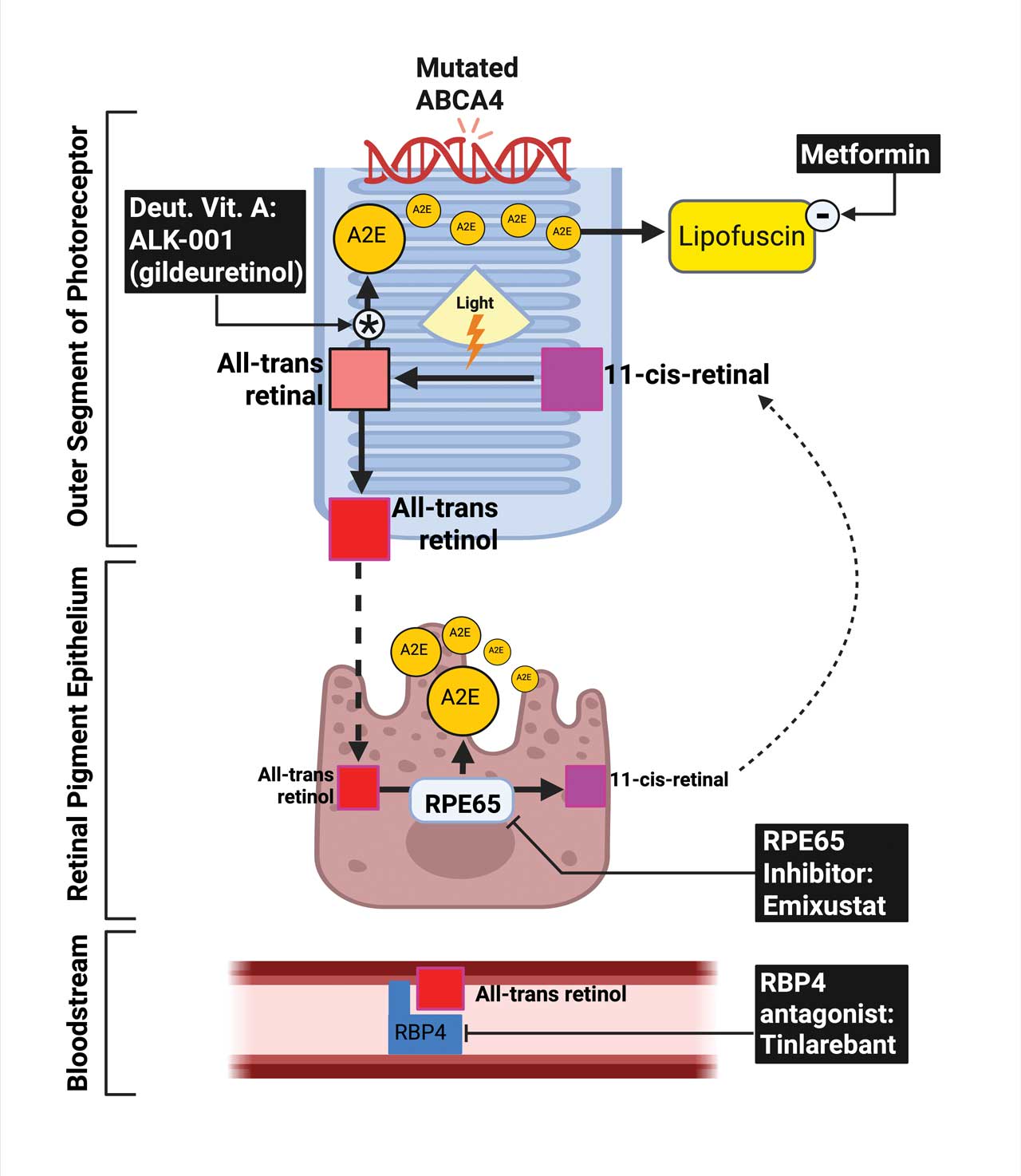
Figure 1. ABCA4 dysfunction in STGD and pharmacologic targets within the visual cycle. In photoreceptor outer segments, the ABCA4 protein facilitates the clearance of all-trans-retinal. Loss-of-function ABCA4 mutations impair this process, resulting in intradiscal accumulation of toxic bisretinoids such as A2E, which are phagocytosed by the retinal pigment epithelium (RPE) and contribute to lipofuscin deposition and RPE toxicity. The visual cycle involves photoisomerization of 11-cis-retinal to all-trans-retinal, followed by reduction to all-trans-retinol, its transfer to the RPE, and reconversion by RPE65 to 11-cis-retinol. Multiple pharmacologic strategies aim to mitigate toxic byproduct formation: ALK-001 (deuterated vitamin A) slows A2E generation; metformin may modulate lipofuscin accumulation; emixustat inhibits RPE65 to reduce chromophore recycling; and tinlarebant (RBP4 antagonist) limits systemic retinol delivery. Together, these interventions target distinct steps in the visual cycle to slow disease progression in STGD.
Oral Therapies Targeting the Retinoid Cycle in STGD
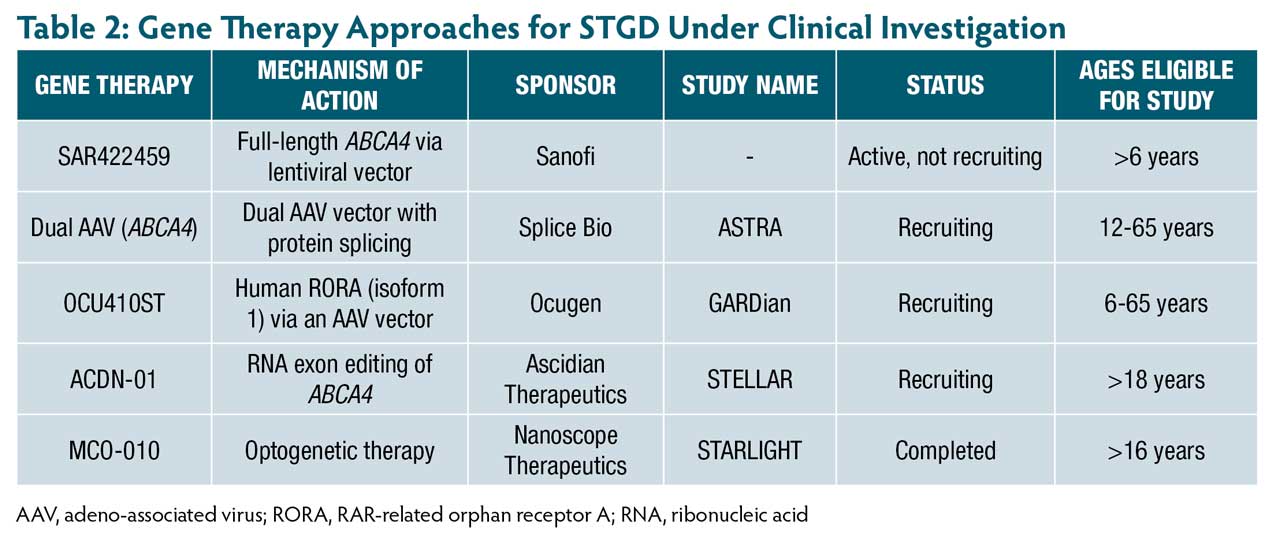
Several clinical trials are underway targeting pathogenic pathways in STGD (Table 1). These therapies aim to reduce toxic bisretinoids accumulation by modulating vitamin A transport or inhibiting enzymes in the visual cycle (Figure 1).
ALK-001 (gildeuretinol; Alkeus Pharmaceuticals)is the leading candidate in development. As a deuterated form of vitamin A, ALK-001 stabilizes the molecule to slow dimerization that generates toxic bisretinoids, thereby reducing A2E accumulation while preserving visual cycle function. The Tolerability and Effects of ALK-001 in STGD (TEASE-1) study was the first in a series of prospective trials evaluating whether modulating vitamin A dimerization can modify the course of STGD.5 In the phase 2 TEASE-1 trial (n=50; ages 18-60), ALK-001 was well tolerated and significantly slowed atrophic lesion progression by 21.6% over 24 months compared to placebo (P<.001).6 Subsequently, TEASE-2 (n=80) is an ongoing clinical trial in subjects with moderate STGD, with results expected in 2025.7 Most recently, TEASE-3 extended the investigation to presymptomatic adolescents (n=7, ages 13-18). Over 24 months, ALK-001 remained well tolerated. Treated participants preserved retinal structure and function, while their older, untreated siblings with symptomatic STGD exhibited progressive decline, highlighting the potential of ALK-001 as a disease-modifying therapy.8 TEASE-4 is an ongoing open-label extension involving participants from all prior TEASE trials to evaluate long-term safety and efficacy.7
Tinlarebant (LBS-008; Belite Bio) is a selective retinol-binding protein 4 (RBP4) antagonist designed to reduce vitamin A delivery to the retina. By reducing RBP4 levels, tinlarebant decreases retinol uptake into RPE, limiting the formation of toxic bisretinoids.9 In a phase 1b/2 trial, tinlarebant demonstrated a favorable safety and tolerability profile in adolescents (ages 12-18) with STGD. At 15 months, results suggested a trend toward slowing the expansion of areas of questionably decreased autofluorescence (QDAF) and stabilizing visual acuity (VA).10 Building on this, 24-months phase 2 data reported favorable structural outcomes. Fundus autofluorescence imaging showed no incident atrophic lesions identified as definitely decreased autofluorescence (DDAF) in 5 of 12 participants (42%). In the remaining participants, the rate of DDAF lesion growth was reduced by approximately 50% compared to matched historical controls (P<.001).11 These results reinforce the potential of tinlarebant as a disease-modifying intervention in early STGD. Advancing this research, the phase 3 DRAGON trial, a global, multicenter, randomized, double-masked, placebo-controlled study (n=104; ages 12-18), is underway, with topline data expected in late 2025.12
Emixustat (ACU-4429; Kubota Vision) is an oral small molecule that inhibits the retinal pigment epithelium–specific 65 kDA protein (RPE65), thereby reducing the production of the visual chromophore (11-cis-retinal) and limiting the accumulation of toxic bisretinoids.13 Despite promising effects in early phase studies,14 in the phase 3 SeaSTAR trial, emixustat did not meet its primary endpoint in reducing macular atrophy progression in STGD subjects.15
Oral Therapies Targeting Cellular Metabolism in STGD
Beyond retinoid cycle modulation, metformin has been considered a potential therapy targeting dysregulated lipid metabolism in STGD. Recent in vitro studies using induced pluripotent stem cell-derived RPE from STGD subjects revealed that ABCA4 deficiency results in a cell-autonomous phenotype marked by intracellular lipid accumulation and photoreceptor outer segment degradation.16 These defects point to lipid metabolism as a therapeutic target. Metformin, an FDA-approved oral agent for type 2 diabetes, enhances lipid metabolism and autophagic flux through activation of AMP-activated protein kinase (AMPK).17 Preclinical evidence in Abca4-/- mouse models demonstrated that metformin reduces lipofuscin buildup and photoreceptor loss, supporting its potential role in STGD.17 The National Eye Institute is currently conducting a clinical trial in STGD (n=56) to evaluate metformin, with the primary endpoint defined as the difference in the growth rate of square root–transformed area of ellipsoid zone loss on optical coherence tomography between the pretreatment phase and the 24-month treatment period.18
Current Landscape of Gene Therapy in STGD
Several gene-based strategies are in development for STGD (Table 2), aiming to restore or modulate retinal function through viral vectors. The following section outlines key therapeutic approaches by vector type and mechanism.
Lentiviral Vector Strategy
SAR422459 (Sanofi) represents one of the earliest direct gene supplementation approaches for STGD using a lentiviral vector to deliver the full-length ABCA4 gene. In a phase 1/2a trial of 22 adult participants, SAR422459 was generally well tolerated, with adverse events primarily related to surgical delivery. No clinically significant changes in visual function were observed. However, 27% of treated eyes showed worsening hypoautofluorescent changes, and 1 high-dose patient demonstrated a notable reduction in macular flecks compared to the untreated eye.19 Long-term follow-up in the ongoing extension study through 2033 will be essential to characterize the safety and clinical relevance of lentiviral gene therapy in STGD.20
Dual AAV Vector Strategy
To overcome the packaging limit of adeno-associated viruses (AAVs), which cannot carry the full-length ABCA4, novel dual AAVs have been developed.21 This approach splits the ABCA4 coding sequence into 2 AAV vectors. Once co-delivered to photoreceptors, the halves undergo protein trans-splicing, mediated by split-inteins, to reconstitute full-length ABCA4 protein.22 Preclinical studies in Abca4-/-mouse and pig models using dual-AAV8 (SB-007; Splice Bio) demonstrated efficient photoreceptor transduction, robust protein reconstitution, and reduced bisretinoid accumulation.22 These findings led to a first-in-human trial, the ongoing phase 1/2 ASTRA study, which was launched in 2025 to evaluate the safety and efficacy of SB-007 in STGD.23
Modifier Gene Therapy Approach
OCU410ST(Ocugen) is a novel modifier gene therapy under investigation for STGD. Instead of replacing ABCA4, OCU410ST delivers human RORA (isoform 1) via an AAV5 vector to regulate retinal homeostasis. Its mechanism targets inflammation, oxidative stress, lipid metabolism, and complement activation.24 In a phase 1 open-label, dose-escalation trial, 9 participants received a single subretinal injection in the worse-seeing eye. OCU410ST was well tolerated. At 6 months, treated eyes showed stable or improved VA, and atrophic lesion growth was 54% slower vs untreated fellow eyes.24 These findings support a phase 2/3 pivotal trial, which has received FDA clearance.25 This gene-agnostic strategy offers a promising alternative to conventional gene replacement.
RNA Editing Strategy
ACDN-01 (Ascidian Therapeutics) is an in vivo RNA exon-editing therapy delivered via subretinal injection by a single vector, designed to correct ABCA4 mutations at the transcript level. The ongoing STELLAR phase 1/2 trial is the first clinical study of this approach in STGD and is currently evaluating safety, tolerability, and efficacy.26,27
Optogenetics
Optogenetics is a gene therapy approach that introduces light-sensitive proteins into retinal cells not typically responsive to light, such as ganglion and bipolar cells, to restore photosensitivity in the absence of functional photoreceptors.28One such therapy, multi-characteristic opsin (MCO-010; Nanoscope Therapeutics), uses an intravitreal AAV2 vector to target bipolar cells. MCO-010 does not require genetic testing, surgery, or external devices such as goggles, offering a more accessible option for patients with advanced retinal degeneration.29 In the phase 2a STARLIGHT trial, MCO-010 was well tolerated and showed sustained BCVA improvements over 48 weeks (reported 12 ETDRS letter improvement and 31-letter gain with magnifiers). This first-in-human study highlights MCO-010’s potential for restoring vision in STGD.30
Stem Cell Replacement Therapy
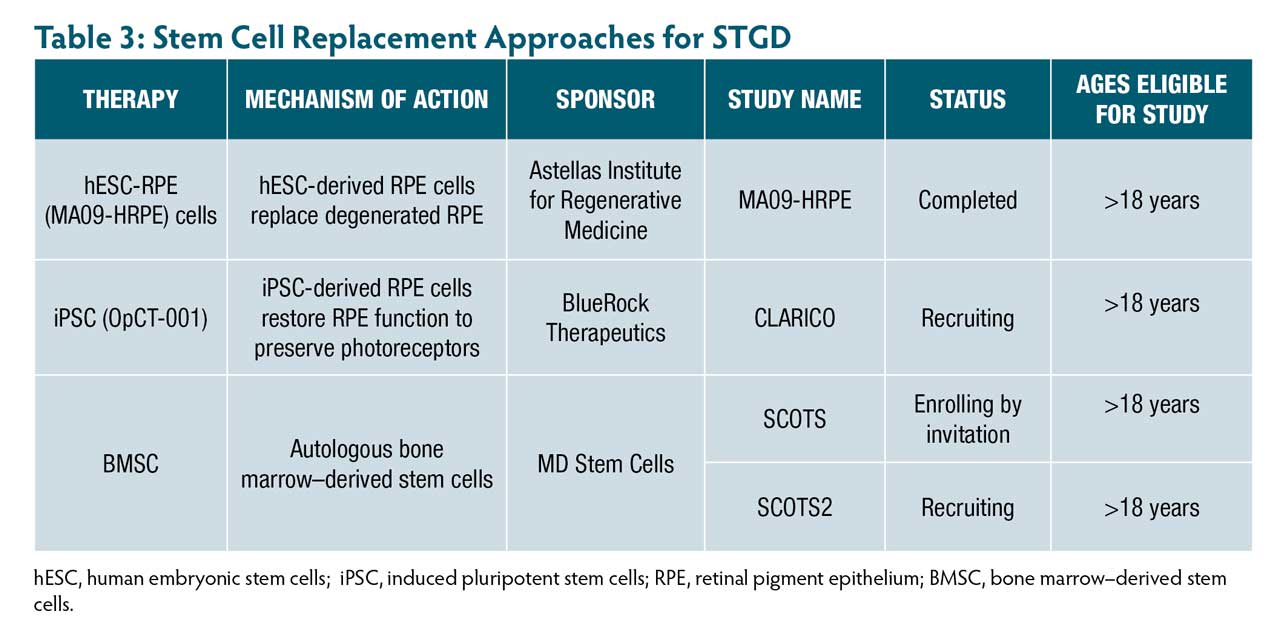
Subretinal transplantation of human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC)-derived RPE has demonstrated long-lasting efficacy in preclinical models (Table 3).31 An early phase human trial by Astellas demonstrated that hESC-RPE (MA09-hRPE) was safe and led to sustained visual improvement in 4 of 10 participants.32
More recently, BlueRock Therapeutics initiated the OpCT-001 trial to evaluate iPSC-derived RPE in humans, with completion expected in 2030.33 In parallel, autologous bone marrow–derived stem cell (BMSC) therapy showed functional improvement in 61.8% of eyes with STGD in the Stem Cell Ophthalmology Treatment Study (SCOTS and SCOTS2), with no adverse events reported.34 The ongoing SCOTS2 trial, through 2027, will further evaluate long-term safety and efficacy.35
Conclusion
Therapeutic innovation in STGD is advancing rapidly, with emerging oral, genetic, and cell-based strategies showing early promise. These approaches reflect a deeper understanding of disease mechanisms and aim to preserve retinal structure and function. While validation in larger trials is pending, current progress marks a critical step toward disease-modifying therapies and sets the stage for transformative impact in managing this inherited macular dystrophy. RP
References
1. Michaelides M, Hunt D, Moore A. The genetics of inherited macular dystrophies. J Med Genet. 2003;40(9):641-650. doi:10.1136/jmg.40.9.641
2. Pas JAAH, Dhooge PPA, Hoyng CB. Clinical classification of Stargardt disease. Graefes Arch Clin Exp Ophthalmol. 2024;262(5):1377-1379. doi:10.1007/s00417-023-06292-x
3. Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Starqardt macular dystrophy. Nat Genet. 1997;15(3):236-246. doi:10.1038/ng0397-236
4. Roborel de Climens A, Tugaut B, Dias Barbosa C, Buggage R, Brun-Strang C. Living with Stargardt disease: insights from patients and their parents. Ophthalmic Genetics. 2021;42(2):150-160. doi:10.1080/13816810.2020.1855663
5. Scholl HP, Shah SM, Kay CN, et al. TEASE: a phase 2 clinical trial assessing the tolerability and effects of oral once-a-day ALK-001 on Stargardt disease. Invest Ophthalmol Vis Sci. 2016;57(12):2685.
6. Scholl HP, DeBartolomeo G, Washington I, Saad L. ALK-001 (C20-D3-Vitamin A) slows the growth of atrophic lesions in ABCA4-related Stargardt disease: results of a phase 2 placebo-controlled clinical trial (TEASE study). Invest Ophthalmol Vis Sci. 2022;63(7):38.
7. Etchison D. Alkeus Pharmaceuticals announces two presentations of oral gildeuretinol data during the 48th annual meeting of the Macula Society being held February 12-15, 2025. February 11, 2025. Accessed September 15, 2025. https://alkeuspharma.com/alkeus-announces-two-presentations-of-oral-gildeuretinol-data/
8. Gorin MB, Saad L, DeBartolomeo G, et al. Gildeuretinol arrested Stargardt disease, the TEASE-3 study. Invest Ophthalmol Vis Sci. 2024;65(7):3310.
9. Kim N, Priefer R. Retinol binding protein 4 antagonists and protein synthesis inhibitors: Potential for therapeutic development. Eur J Med Chem. 2021;226:113856. doi:10.1016/j.ejmech.2021.113856
10. Grigg JR, Chen FK, Chen TC, et al. A phase 1b/2 study of the safety and tolerability of tinlarebant in adolescent patients affected by Stargardt disease—15-month preliminary data. Invest Ophthalmol Vis Sci. 2023;64(8):2597.
11. Grigg JR, Chen F, Chen TC, et al. Safety, tolerability, and efficacy of tinlarebant from the 24-month phase 2 study in adolescent patients affected by Stargardt disease. Invest Ophthalmol Vis Sci. 2024;65(7):1026.
12. Belite Bio announces interim analysis results from the pivotal global phase 3 DRAGON trial of tinlarebant in adolescent stargardt disease subjects. News release. February 27, 2025. Accessed September 15, 2025. https://investors.belitebio.com/news-releases/news-release-details/belite-bio-announces-interim-analysis-results-pivotal-global/
13. Kubota R, Gregory J, Henry S, Mata NL. Pharmacotherapy for metabolic and cellular stress in degenerative retinal diseases. Drug Discov Today. 2020;25(2):292-304. doi:10.1016/j.drudis.2019.11.013
14. Kubota R, Birch DG, Gregory JK, Koester JM. Randomised study evaluating the pharmacodynamics of emixustat hydrochloride in subjects with macular atrophy secondary to Stargardt disease. Br J Ophthalmol. 2022;106(3):403-408. doi:10.1136/bjophthalmol-2020-317712
15. National Health Service, Health Research Authority. The SeaSTAR study—amendment 1. March 18, 2019. Accessed September 15, 2025. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/the-seastar-study-amendment-1/
16. Farnoodian M, Bose D, Khristov V, et al. Cell-autonomous lipid-handling defects in Stargardt iPSC-derived retinal pigment epithelium cells. Stem Cell Reports. 2022;17(11):2438-2450. doi:10.1016/j.stemcr.2022.10.001
17. Farnoodian M, Barone F, Boyle M, et al. Metformin attenuates the hallmarks of Stargardt disease. Invest Ophthalmol Vis Sci. 2023;64(8):476.
18. National Eye Institute. Oral metformin for treatment of ABCA4 retinopathy (Stargardt disease). December 16, 2024. Accessed September 15, 2025. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/stargardt-disease/oral-metformin-treatment-abca4-retinopathy
19. Parker MA, Erker LR, Audo I, et al. Three-year safety results of SAR422459 (EIAV-ABCA4) gene therapy in patients with ABCA4-associated Stargardt disease: an open-label dose-escalation phase 1/2a clinical trial, Cohorts 1-5. Am J Ophthalmol. 2022;240:285-301. doi:10.1016/j.ajo.2022.02.013
20. An open label study to determine the long-term safety, tolerability and biological activity of SAR422459 in patients with Stargardt’s macular degeneration. Clinicaltrials.gov identifier: NCT01736592. Updated June 12, 2025. Accessed September 15, 2025. https://clinicaltrials.gov/study/NCT01736592
21. Trapani I. Dual AAV Vectors for Stargardt Disease. In: Boon CJF, Wijnholds J, eds. Retinal Gene Therapy: Methods and Protocols. Springer; 2018:153-175. doi:10.1007/978-1-4939-7522-8_11
22. Favre D, Di Scala M, Aisa I, et al. Preclinical safety and efficacy of intein-based dual-AAV gene therapy for Stargardt disease. Invest Ophthalmol Vis Sci. 2024;65(7):6089.
23. SpliceBio announces first patient dosed in phase 1/2 ASTRA study of SB-007, a dual-AAV gene therapy for Stargardt disease. News release. March 13, 2025. Accessed September 15, 2025. https://splice.bio/splicebio-announces-first-patient-dosed-in-phase-1-2-astra-study-of-sb-007-a-dual-aav-gene-therapy-for-stargardt-disease/
24. Maldonado RS, Vajzovic L, Bakall B, et al. Safety and efficacy of OCU410ST for the treatment of Stargardt Disease: phase 1/2 Study Update. Presented at: Association for Research in Vision and Ophthalmology (ARVO) 2025 meeting; May 4-8, 2025; Salt Lake City, Utah.
25. Ocugen announces US FDA clearance of investigational new drug amendment to initiate phase 2/3 pivotal confirmatory clinical trial of OCU410ST—modifier gene therapy candidate for Stargardt disease. News release. June 16, 2025. Accessed September 15, 2025. https://ir.ocugen.com/news-releases/news-release-details/ocugen-inc-announces-us-fda-clearance-investigational-new-drug/
26. Foundation Fighting Blindness. Ascidian to launch clinical trial for Stargardt disease RNA editing therapy. February 1, 2024. Accessed September 15, 2025. https://www.fightingblindness.org/news/ascidian-to-launch-clinical-trial-for-stargardt-disease-rna-editing-therapy-882
27. ACDN-01-001: Open-label, single ascending dose study to evaluate the safety, tolerability, and preliminary efficacy of subretinal ACDN-01 in participants with ABCA4-related retinopathy. Clinicaltrials.gov identifier: NCT06467344. Updated July 1, 2025. Accessed September 15, 2025. https://clinicaltrials.gov/study/NCT06467344
28. Kulbay M, Tuli N, Akdag A, Kahn Ali S, Qian CX. Optogenetics and targeted gene therapy for retinal diseases: unravelling the fundamentals, applications, and future perspectives. J Clin Med. 2024;13(14):4224. doi:10.3390/jcm13144224
29. Nanoscope Therapeutics initiates rolling submission of biologics license application to FDA for MCO-010, the first gene-agnostic therapy to treat retinitis pigmentosa. News release. July 14, 2025. Accessed September 15, 2025. https://nanostherapeutics.com/2025/07/14/nanoscope-therapeutics-initiates-rolling-submission-of-biologics-license-application-to-fda-for-mco-010-the-first-gene-agnostic-therapy-to-treat-retinitis-pigmentosa/
30. Mahajan VB. Longitudinal BCVA analysis of patients with Stargardt disease and macular degeneration treated with MCO-010, a mutation-agnostic optogenetic therapy: 48-week results from a phase 2a clinical trial (STARLIGHT). Invest Ophthalmol Vis Sci. 2024;65(7):5266.
31. Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27(9):2126-2135. doi:10.1002/stem.149
32. Subretinal transplantation of hESC derived RPE (MA09-hRPE) cells in patients with Stargardt’s macular dystrophy. Clinicaltrials.gov identifier: NCT01345006. Updated October 10, 2024. Accessed September 15, 2025. https://www.clinicaltrials.gov/study/NCT01345006
33. A study to investigate the safety of OpCT-001 in adults who have primary photoreceptor disease (CLARICO). Clinicaltrials.gov identifier: NCT06789445. Updated June 24, 2025. Accessed September 15, 2025. https://clinicaltrials.gov/study/NCT06789445
34. Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow–derived stem cells in the treatment of stargardt disease. Medicines (Basel). 2021;8(2):10. doi:10.3390/medicines8020010
35. Bone Marrow Derived Stem Cell Ophthalmology Treatment Study II. Clinicaltrials.gov identifier: NCT03011541. Updated March 20, 2025. Accessed September 15, 2025. https://clinicaltrials.gov/study/NCT03011541