Since the approval of the first retinal gene therapy, voretigene neparvovec (Luxturna; Spark Therapeutics) for RPE65-associated retinal degeneration in 2017, the landscape of inherited retinal disease (IRD) has markedly changed. Genetic testing has become the standard of care for monogenetic IRD,1 and retina specialists have had to make continual efforts to stay up to date in what is arguably the most rapidly evolving field of ophthalmology, the field of retinal genetics and gene therapy. Given the explosion in this field in the last decade, it would be challenging to give a comprehensive overview of all the retinal gene therapy clinical trials for IRD. This brief article will share updates from a selection of trials highlighting the following IRDs: retinitis pigmentosa (RP), focusing on augmentation gene therapy for RPGR-associated X-linked RP (XLRP) and a gene-agnostic optogenetics approach for RP, Stargardt disease, Leber congenital amaurosis (LCA), and X-linked retinoschisis (XLRS).
Augmentation Trials for RPGR- Associated Retinitis Pigmentosa
Botaretigene sparoparvovec (bota-vec; Johnson & Johnson) is an investigational adeno-associated virus vector type 5 (AAV5) gene therapy being studied for the treatment of XLRP due to variants in the RP GTPase regulator (RPGR) gene.2,3 The LUMEOS study3 was a phase 3 randomized, controlled, multicenter clinical trial evaluating the efficacy and safety of bilateral subretinal administration of bota-vec in patients with XLRP due to variants in the RPGR gene.3 The primary outcome was the change from baseline to week 52 in vision-guided mobility assessment (VMA) as measured by the ability to navigate through a VMA maze.4 At week 52, the primary endpoint (VMA) was not statistically significant but was directionally supportive.5
The secondary endpoints assessed changes after treatment administration in retinal function, functional vision, and visual function.5 The study demonstrated improvement in several secondary endpoints, including data from all 3 visual domains.5 The safety profile of bota-vec was as expected and manageable, with no new safety signals noted in the phase 3 study.5 Overall, 100% of participants in the pooled treated group experienced at least 1 treatment- emergent adverse event (TEAE), with the majority (86%) being mild or moderate.5 Just over half (53%) had ≥1 TEAE considered to be related to bota-vec, and 98% had ≥1 TEAE related to the surgical procedure.5
Beacon Therapeutics is a clinical-stage biotechnology company with a lead program, laru-zova (laruparetigene zovaparvovec), a gene therapy currently being investigated for the treatment of patients with XLRP. X-linked retinitis pigmentosa is an IRD that predominantly affects males, typically caused by mutations in the RPGR gene. The mutations, which affect approximately 1 in 25,000 males in the United States, Europe, and Australia, result in progressive photoreceptor loss over time and visual dysfunction beginning in childhood, eventually leading to blindness and impacting quality of life with no approved treatments. Laru-zova has the potential to restore the natural function of both rods and cones in XLRP by delivering a functional copy of the RPGR ORF15 gene designed to produce the full-length protein.
DAWN is an open-label study of laru-zova in the fellow eye of male patients with XLRP who have previously been treated with an AAV vector-based gene therapy delivering the full-length RPGR protein.6 The study aims to assess 2 dose levels of laru-zova for efficacy, safety, and tolerability in the untreated eye of participants who previously received gene therapy for XLRP. Interim safety and efficacy results from the phase 2 DAWN trial demonstrated laru-zova was generally well tolerated by all DAWN participants evaluated at 6 months or beyond and initial data showed promising improvements in visual function across several key measures. Currently the pivotal phase 2/3 VISTA trial of laru-zova for patients with XLRP has completed enrollment.
VISTA is a phase 2/3, randomized, controlled, masked, multicenter pivotal study evaluating the efficacy, safety and tolerability of laru-zova in 2 study groups compared to an untreated control group.7 The study will evaluate the proportion of participants with a 15-or-more-letter increase from baseline in low-luminance visual acuity (LLVA) and additional measures of functional vision.
Gene Therapy for ABCA4-Associated Stargardt Disease
Ascidian Therapeutics is a biotechnology company focused on RNA exon editing technology. ACDN-01 is an in vivo RNA exon editor delivered by a single vector for Stargardt disease and is currently being evaluated in the phase 1/2 STELLAR study.8 ACDN-01 directly corrects ABCA4 messenger RNA, replacing mutated RNA with wild type using the cell’s own splicing machinery. Unlike CRISPR or ADAR technology, Ascidian’s platform edits thousands of bases of RNA in a single reaction, allowing for treatment of a much larger patient population. It has demonstrated efficient, durable in vivo RNA exon editing in nonhuman primate (NHP) retina and ex vivo RNA exon editing in human retinal explants. In NHP studies, ACDN-01 not only successfully edited ABCA4 RNA, but also generated full-length, functional ABCA4 protein. ACDN-01 has been granted Rare Pediatric Disease and Fast Track Designation by the US Food and Drug Administration (FDA). ACDN-01 is the first ever RNA exon editor to enter clinical development and the first RNA editing therapeutic of any kind to be cleared for clinical investigation in the United States before being approved for trials in any other country.
The STELLAR trial is a phase 1/2 study of ACDN-01 designed to show safety as well as early signals of efficacy. The primary endpoints are focused on safety. Efficacy endpoints will evaluate the change in rate of atrophy progression as well as retinal function. Enrollment is under way and data will be reported at future meetings as they are generated. The study is US-based with 10 sites, most of which accept external referrals. In addition, there is a separate prescreening study, which is open to both pediatric and adult patients.
SpliceBio, a clinical-stage genetic medicines company, has developed a proprietary protein-splicing intein platform using dual AAV vectors that enables the delivery of genes too large for conventional AAV vector systems. The company is currently leveraging this technology to develop SB-007, a genetic treatment for Stargardt disease caused by dysfunction in the ABCA4 gene.9 SB-007 consists of dual AAV8 vectors carrying the ABCA4 gene, split into 2 segments and fused to engineered intein sequences that mediate protein trans-splicing to reconstitute full-length, functional ABCA4 in target cells.10,11 When administered in vivo via subretinal injection, SB-007 demonstrated high-level transduction in the retina in photoreceptor cells and dose-dependent pharmacologic activity, as measured by the reduction of A2E levels in mouse and rat. In Abca4 knockout mice and rats and in wild-type models (pig and NHP), full reconstitution of ABCA4 protein was observed. In a large, 6-month, pivotal GLP toxicology NHP study, SB-007 was well tolerated at all tested doses for up to 6 months. SpliceBio received an approval of their IND in December 2024.10,11 An observational study called POLARIS was initiated in March 2024 with the objective of monitoring disease progression in subjects with Stargardt disease type 1 caused by biallelic autosomal recessive mutations in the ABCA4 gene.11,12 In March 2025, SpliceBio announced that it had dosed the first patient in the phase 1/2 ASTRA study of SB-007 for the treatment of Stargardt disease. The ASTRA study will evaluate the safety, tolerability, and preliminary efficacy of subretinal SB-007 administration, with the goal of determining the optimal dose in subjects with Stargardt disease type 1.9,11
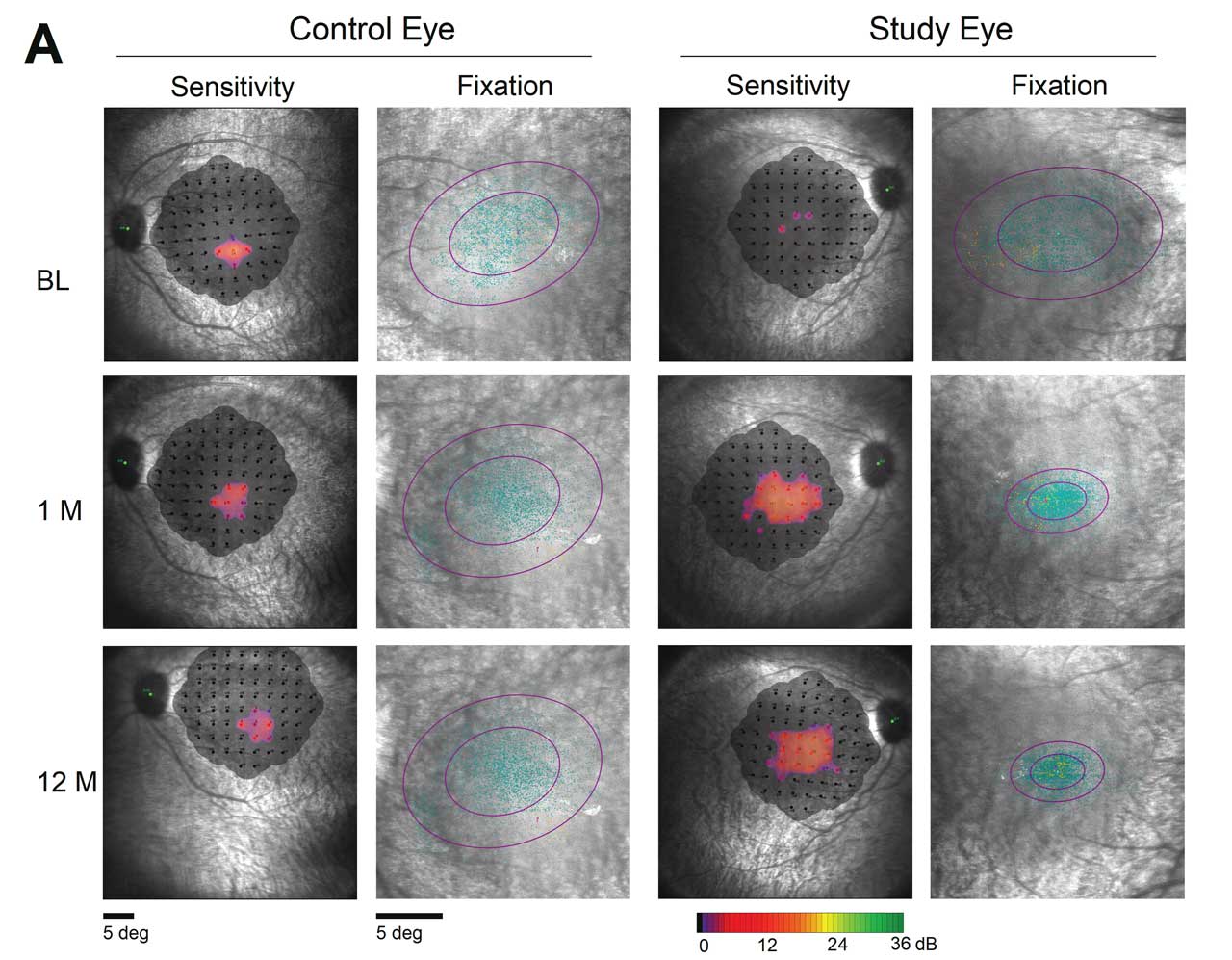
Figure 1. Greater than 18-fold improvement was noted in macular sensitivity in patient treated with OPGx-LCA5, according to 12-month data presented at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting in May 2025.
Gene Therapy for Leber Congenital Amaurosis
Atsena Therapeutics recently published 12-month safety and efficacy data from a phase 1/2 clinical trial evaluating the subretinal gene therapy ATSN-101 in patients with LCA type 1 (LCA1), caused by biallelic mutations in GUCY2D.13
In the dose-escalation phase (part A), 3 cohorts of 3 adult patients each were treated with 3 ascending dose levels of ATSN-101. In the dose-expansion phase (part B), an additional adult cohort and a pediatric cohort, including 3 patients each, were treated at the high dose from part A. All adverse events were grade 1-2 in severity, the majority of which were related to the surgical procedure, and no serious adverse events were related to the study drug. For patients who received high-dose ATSN-101, the mean change in dark-adapted full-field stimulus test (FST) was 20.3 dB in treated eyes, representing a 100-fold improvement, compared with 1.1 dB in untreated eyes. In 2 of 9 eyes treated at the high dose, improvements in FST exceeded 40 dB (10,000-fold). Modest improvements in BCVA (-0.16 logMAR or 8 letters) were also observed, and 3 of 6 high-dose patients who performed the multiluminance mobility test (MLMT) achieved the maximum score in the treated eye at 12 months posttreatment. Atsena Therapeutics is currently moving forward with plans for a phase 3 trial.
The Opus Genetics pipeline consists of 7 AAV-based gene therapies, each targeting a specific IRD. OPGx-LCA5 is Opus’s most advanced gene therapy program, for LCA type 5 (LCA5). It uses an AAV8 vector to deliver a functional LCA5 gene to the outer retina and has the same CβA promoter technology as voretigene. A phase 1/2 study of OPGx-LCA5 is ongoing, with 12-month data available from 3 adult patients in the low-dose cohort (1E10 vg/eye).14 Each patient had severe LCA5 at baseline, with limited but detectable photoreceptors and disease progression to the central retina. At 12 months, all treated patients identified more objects compared to baseline on the Multi-Luminance Orientation and Mobility Test (MLoMT), a virtual reality-based test designed to measure changes in functional vision. Two out of 3 patients showed clinically meaningful improvement in MLoMT with a 3-object recognition threshold (ORT) or more improvement; the third patient went from being unable to complete the MLoMT course to being able to complete it at 12 months. There was a 3.5-line improvement in visual acuity across the 3 patients at 12 months.
Additionally, full-field stimulus testing demonstrated larger improvements in sensitivity from baseline in treated eyes, with a 0.86 log improvement at 12 months vs 0.16 log units for control eyes. Microperimetry data were collected from 1 patient (not possible in the other 2 participants due to poor fixation). At 12 months, fixation stabilized and shifted toward the foveal center, suggesting improved central vision with improved fixation (Figure 1). OPGx-LCA5 was well tolerated with no reports of dose-limiting toxicities or serious adverse events out to 12 months.
Three pediatric patients have also been treated with OPGx-LCA5, with initial data expected in 2025. Opus is also developing an investigational gene therapy for BEST1 disease and has plans to initiate a phase 1/2 study in 2025, with preliminary data expected in early 2026.
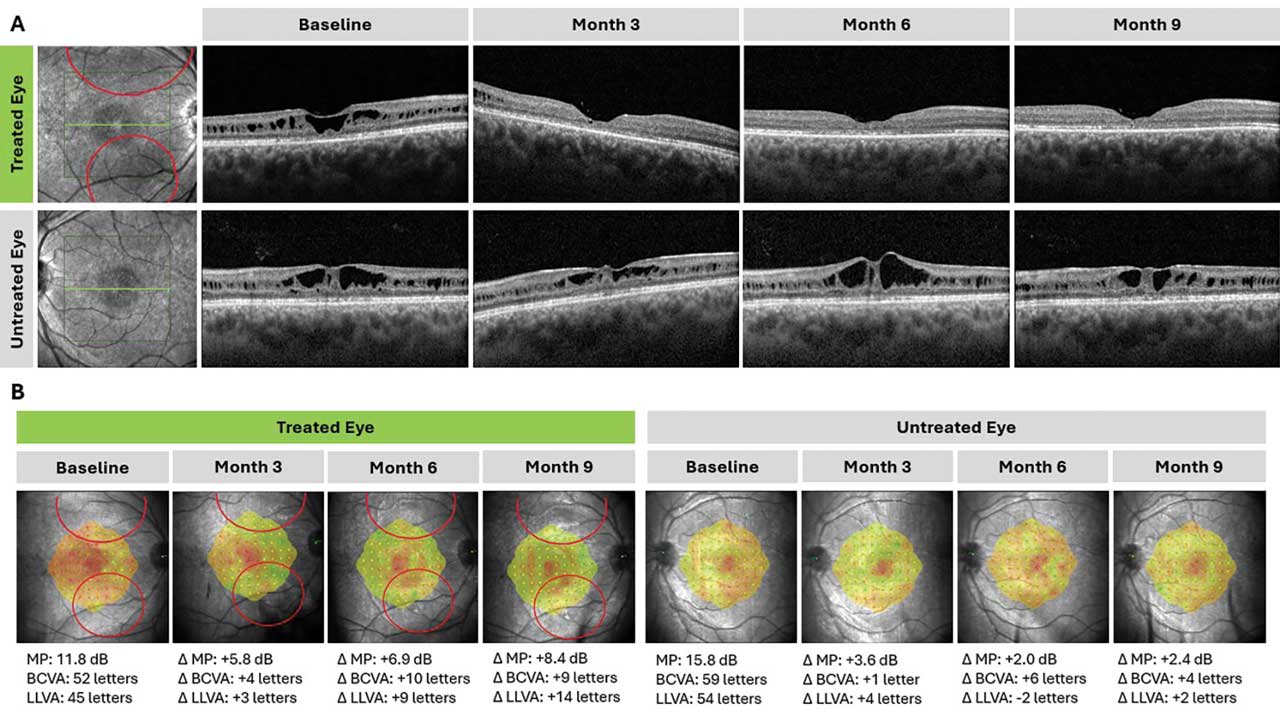
Figure 2. Structural and functional changes in a patient treated with ATSN-201 (Atsena Therapeutics). ATSN-201 was delivered by subretinal injection to the right eye in 2 blebs (bleb locations shown in red). Foveal schisis closure was observed in the treated eye by month 3 and has persisted through at least month 9, demonstrating the durability of the treatment effect; schisis closure was not observed in the untreated eye at any time point (A). The treated eye also demonstrated functional improvements on microperimetry (MP), best-corrected visual acuity (BCVA), and low-luminance visual acuity (LLVA) relative to the untreated eye (B). MP threshold was calculated as the average of 5 loci with the lowest sensitivity at baseline, using a 19 dB cutoff and including additional loci if there was a tie.
Gene Therapy for X-Linked Retinoschisis
The LIGHTHOUSE study, a phase 1/2 clinical trial sponsored by Atsena Therapeutics, is evaluating subretinal gene therapy ATSN-201 for the treatment of XLRS. ATSN-201 leverages AAV.SPR, the company’s novel spreading capsid, to achieve therapeutic levels of gene expression in photoreceptors of the central retina while avoiding the surgical risks of foveal detachment. Part A of the study is evaluating the safety and tolerability of 3 dose levels of ATSN-201, administered to one eye via subretinal injection, in 3 cohorts of 3 adult patients each.
According to data reported in May 2025, ATSN-201 has been well tolerated in all 9 patients to date, with no reported serious adverse events related to the study drug.15 Most adverse events were grade 1-2 in severity and related to the surgical procedure. The only reported serious adverse event, a fever of unknown origin with negative workup, occurred 7 months posttreatment and was considered unrelated to ATSN-201 or the surgical procedure. There were no dose-limiting toxicities, and no patients discontinued the study. Preliminary evidence of efficacy was observed at all 3 dose levels of ATSN-201. Seven of 9 treated eyes had closure of foveal schisis; for the 2 eyes that did not demonstrate foveal schisis closure, 1 had blebs placed further in the periphery and the other developed an epiretinal membrane following intraoperative laser. In contrast, none of the untreated eyes demonstrated closure of foveal schisis. Improvements in visual function were generally observed in eyes with structural improvements. On microperimetry (MP), 6 of 9 (67%) treated eyes improved by ≥7 dB in the 5 or more loci with the lowest sensitivity at baseline. Statistically significant improvements in BCVA and LLVA were also reported. Figure 2 shows an example of structural and functional improvements for 1 patient through 9 months posttreatment.
Enrollment in part B of the study is currently under way and will evaluate 9 additional adult patients and 3 pediatric patients with XLRS. The adult cohort will be randomized into 3 arms: (1) low volume, (2) high volume, and (3) control. Patients in the control arm will have the option to receive treatment after being observed for 12 months.
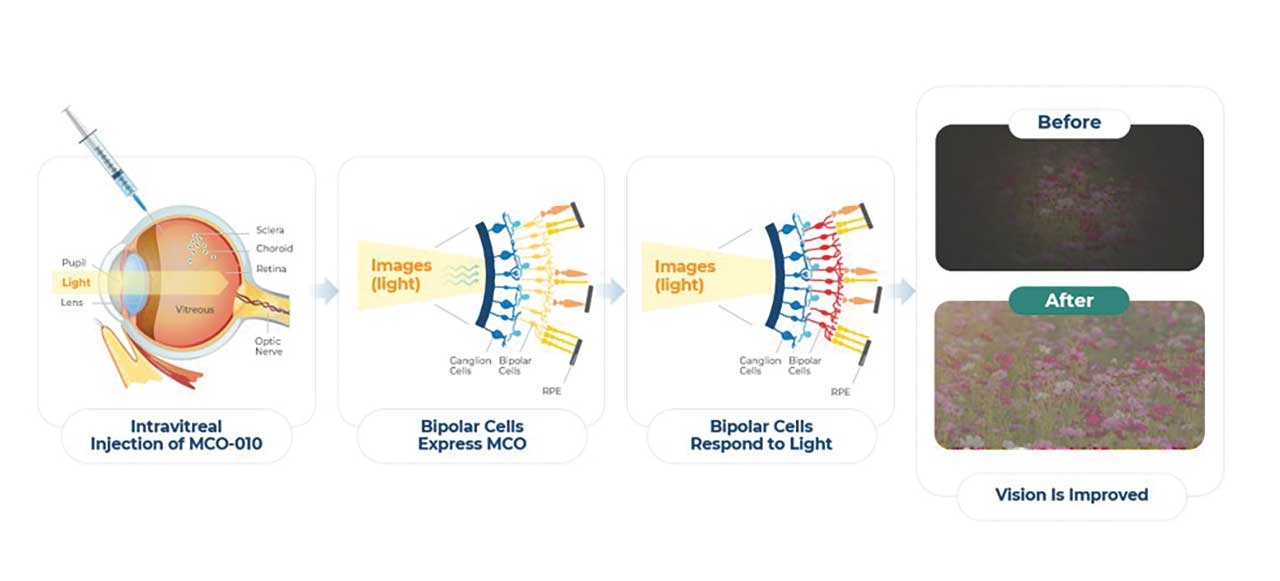
Figure 3. MCO-010 is intravitreally delivered via AAV2 as a multicharacteristic opsin (MCO) transgene to transduce bipolar cells to express a photosensitive opsin protein.
Gene-Agnostic Programs
Nanoscope Therapeutics is developing gene-agnostic, vision-restoring optogenetic therapy for millions of patients blinded by retinal degenerative diseases. MCO-010 is delivered via AAV2 as a multicharacteristic opsin (MCO) transgene by a one-time intravitreal injection (IVT) to transduce bipolar cells to express a highly photosensitive opsin protein, restoring vision in patients despite permanent photoreceptor loss (Figure 3). Following the positive outcomes from the RESTORE phase 2b/3 multicenter, randomized controlled clinical trial,16 which demonstrated statistically significant vision improvements of 0.3 LogMAR (equivalent to a 3-line gain on the ETDRS chart) and durable efficacy sustained over 2.5 years in patients with RP, a rolling Biologics License Application (BLA) submission to the FDA has been initiated in June 2025. If approved, MCO-010 has the potential to be the standard of care for RP patients, administered as a one-time, in-office injection without the need for genetic testing. The company has also shown promising results in the STARLIGHT phase 2 clinical trial of MCO-010 in Stargardt disease17 and plans to initiate a phase 3 registrational trial in 2025. Preclinical programs include LCA, in investigational new drug (IND)-enabling studies, as well as an IND-ready asset for geographic atrophy.
Conclusion
Now more than ever, it is imperative that retina specialists stay apprised of the landscape of IRD gene therapy trials to be able to provide the best, most up-to-date clinical management for the IRD patient population. Whether patients with a suspected IRD are immediately referred to an IRD specialist, or if genetic testing is begun in a general retina practice remains a personal practice preference, however the more information retina specialists have regarding the clinical trial landscape, the more useful conversations can be for patients around applicable clinical trials or potential treatment. RP
References
1. Stone EM, Aldave AJ, Drack AV, et al. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology task force on genetic testing. Ophthalmology. 2012;119(11):2408-2410. doi:10.1016/j.ophtha.2012.05.047
2. Gene therapy for X-linked retinitis pigmentosa (XLRP) retinitis pigmentosa GTPase regulator (RPGR). ClinicalTrials.gov identifier: NCT03252847. Updated January 8, 2025. Accessed September 8, 2025. https://www.clinicaltrials.gov/ct2/show/NCT03252847
3. Michaelides M, Besirli CG, Yang Y, et al. Phase 1/2 AAV5-hRKp.RPGR (botaretigene sparoparvovec) gene therapy: safety and efficacy in rpgr-associated x-linked retinitis pigmentosa. Am J Ophthalmol. 2024;267:122-134. doi:10.1016/j.ajo.2024.05.034
4. Gene therapy trial for the treatment of X-linked retinitis pigmentosa associated with variants in the RPGR gene. ClinicalTrials.gov identifier: NCT04671433. Updated April 25, 2025. Accessed September 8, 2025. https://www.clinicaltrials.gov/ct2/show/NCT04671433
5. Clark M, Besirli C, Wong P, et al. Update on bota-vec efficacy and safety data in patients with RPGR-XLRP. Oral presentation presented at: FFB Retinal Therapeutics Innovation Summit 2025; May 2, 2025; Salt Lake City, UT, USA.
6. A phase 2, open-label, multicenter study comparing two doses of AGTC-501 (laruparetigene zovaparvovec; DAWN) in male participants with X-linked retinitis pigmentosa (previously treated with AAV-based RPGR gene therapy). ClinicalTrials.gov identifier: NCT06275620. Updated October 30, 2024. Accessed September 8, 2025.
7. A phase 2/3, randomized, controlled, masked, multicenter pivotal study evaluating two doses of laru-zova (AGTC-501/laruparetigene zovaparvovec) versus untreated control in male participants with X-linked retinitis pigmentosa (VISTA). ClinicalTrials.gov identifier: NCT04850118. Updated February 11, 2025. Accessed September 8, 2025. https://clinicaltrials.gov/study/NCT04850118
8. ACDN-01-001: open-label, single ascending dose study to evaluate the safety, tolerability, and preliminary efficacy of subretinal ACDN-01 in participants with ABCA4-related retinopathy (STELLAR). ClinicalTrials.gov identifier: NCT06467344. Updated June 27, 2025. Accessed September 8, 2025. https://clinicaltrials.gov/study/NCT06467344
9. SpliceBio. SpliceBio announces first patient dosed in Phase 1/2 ASTRA study of SB-007, a dual-AAV gene therapy for Stargardt disease [Press release]. March 13, 2025. https://splice.bio/splicebio-announces-first-patient-dosed-in-phase-1-2- astra-study-of-sb-007-a-dual-aav-gene-therapy-for-stargardt-disease/
10. Favre D, Di Scala M, Aisa I, et al. Preclinical safety and efficacy of intein-based dual-AAV gene therapy for Stargardt disease. Invest Ophthalmol Vis Sci. 2024;65:6089. https://iovs.arvojournals.org/article.aspx?articleid=2800129
11. Girach A, Favre D. Intein-based dual-AAV gene therapy for Stargardt disease. Presented at: 9th Annual Retinal Cell and Gene Therapy Innovation Summit; May 3, 2024; Seattle, WA.
12. SpliceBio. SpliceBio announces U.S. FDA IND clearance of SB-007 to commence Phase 1/2 clinical study in patients with Stargardt disease. [Press release]. December 12, 2024. https://splice.bio/splicebio-announces-u-s-fda-ind-clearance-of- sb-007-to-commence-phase-1-2-clinical-study-in-patients-with-stargardt-disease/
13. Yang P, Pardon LP, Ho AC, et al. Safety and efficacy of ATSN-101 in patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D: a phase 1/2, multicentre, open-label, unilateral dose escalation study. Lancet. 2024;404(10456):962-970. doi:10.1016/S0140-6736(24)01447-8
14. Opus Genetics. Opus Genetics announces presentation of OPGX-LCA5 gene therapy data at ARVO; 12-month phase 1/2 results support potential to restore to meaningful vision. May 5, 2025. Accessed September 8, 2025. https://ir.opusgtx.com/press-releases/detail/484/opus-genetics-announces-presentation-of-opgx-lca5-gene-therapy-data-at-arvo-12-month-phase-12-results-support-potential-to-restore-to-meaningful-vision
15. Atsena Therapeutics announces positive clinical data from part A of phase 1/2 trial evaluating ATSN-201 gene therapy to treat X-linked retinoschisis (XLRS). News release. May 19, 2025. Accessed September 8, 2025. https://www.atsenatx.com/press-release/atsena-therapeutics-announces-positive-clinical-data-from-part-a-of-phase-i-ii-trial-evaluating-atsn-201-gene-therapy-to-treat-x-linked-retinoschis/
16. A phase 2b randomized, double-masked, sham-controlled study to evaluate the efficacy and safety of intravitreal injection of MCO-010 optogenetic therapy in adults with retinitis pigmentosa (RESTORE). ClinicalTrials.gov identifier: NCT04945772. Updated March 22, 2024. Accessed September 8, 2025. https://clinicaltrials.gov/study/NCT04945772
17. Safety and effects of a single intravitreal injection of vMCO-010 optogenetic therapy in subjects with Stargardt disease (STARLIGHT). ClinicalTrials.gov identifier: NCT05417126. Updated November 9, 2023. Accessed September 8, 2025. https://clinicaltrials.gov/study/NCT05417126









