Gene therapy delivered via suprachoroidal injection may one day offer an office-based option for treating retinal diseases, with early data showing durable effect and fewer complications than traditional approaches. During a session Friday at the 2025 Retina World Congress in Fort Lauderdale, David S. Boyer, MD, outlined the advantages and early results of suprachoroidal gene therapy.
The suprachoroidal space is a potential space between the sclera and choroid, and a drug or agent injected into this space flows posteriorly toward the macula due to a natural pressure gradient between the anterior and posterior segments, explained Dr. Boyer, an adjunct clinical professor at the USC Keck School of Medicine and a partner at Retina Vitreous Associates of Los Angeles. “Preclinical studies have shown durable high levels of drug in the targeted retina, retinal pigment epithelium, and choroid, and lower exposure to the anterior chamber, iris, ciliary body, and lens,” he said. “This may decrease off-target binding and immune responses.”
Instruments to Enter the Subretinal Space
Catheter-Based Instruments
-
Oxulumis Subretinal Injection Device (Oxulumis Ltd)
-
Everads Suprachoroidal Delivery System (Everads Therapy)
-
Orbit Subretinal Delivery System (Gyroscope Therapeutics)
Microneedle-Based Instruments
-
SCS Microinjector (Clearside Biomedical)
-
Bella-vue (Uneedle)
-
Standard hypodermic needle
Suprachoroidal delivery has attracted increasing attention in gene therapy for retinal diseases because of its targeted delivery profile and feasibility as an in-office procedure, Dr. Boyer said. Several devices have been developed to facilitate this delivery, but the SCS Microinjector (Clearside Bio), which uses a fine-tipped needle to access the space, is most commonly used, he said. “Gene therapies through the suprachoroidal space work,” Dr. Boyer said. “It certainly is attractive, and it’s an office procedure.”
Dr. Boyer noted that suprachoroidal delivery is being used in the ongoing clinical development of ABBV-RGX-314, a one-time gene therapy under investigation for nonproliferative diabetic retinopathy (NPDR), including severity and progression to vision-threatening complications, and neovascular age-related macular degeneration (nAMD). Dr. Boyer is a consultant for AbbVie and RegenxBio, which are jointly developing ABBV-RGX-314.
In the phase 2 ALTITUDE trial, 70.8% of patients treated with a single suprachoroidal injection of AABV-RGX-314 achieved an improvement of at least 1 step on the Diabetic Retinopathy Severity Scale (DRSS) at a year following treatment. No patients experienced a 2-step worsening, and the therapy was well tolerated. In contrast, the control group saw 37.5% of patients worsen by 2 DRSS steps or more. More importantly the treatment reduced vison-threatening events from 37.5% in sham to 4.2% in cohort 2 (5×10¹¹ genomic copies per eye [GC/eye]) in patients with DRSS scores between 47 and 53 (Figure 1). In January, AbbVie and RegenxBio reported that they are planning a phase 3 clinical program for AABV-RGX-314 in NPDR, which will use the SCS Microinjector.
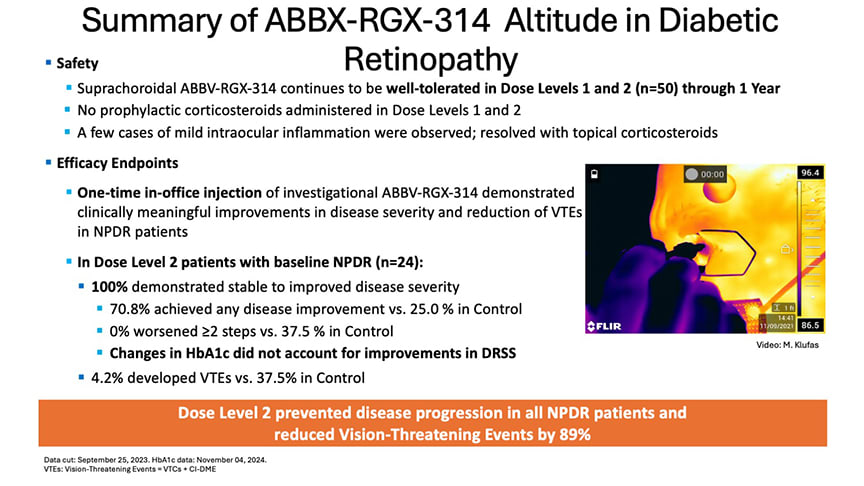
Figure 1. ALTITUDE data demonstrates ABBV-RGX-314’s ability to stabilize or improve nonproliferative diabetic retinopathy (NPDR) following a suprachoroidal injection. Dose level 2 prevented disease progression in all NPDR patients and reduced vision-threatening events by 89% compared to the control group.
In the phase 2 AAVIATE trial for nAMD, ABBV-RGX-314 delivered via a single suprachoroidal injection showed promising results in reducing treatment burden. Across dose cohorts, patients experienced a marked reduction in their annualized anti-VEGF injection rate: those in cohort 1 (2.5×10¹¹ GC/eye) saw a mean reduction of 66.7%, while cohort 2 (5×10¹¹ GC/eye) achieved a 75% reduction, and cohort 3 (1×10¹² GC/eye) reported an 85.7% reduction compared to their injection frequency prior to gene therapy (Figure 2). ABBV-RGX-314 was generally well tolerated in AAVIATE, with most ocular adverse events being mild or moderate and responsive to topical corticosteroids.
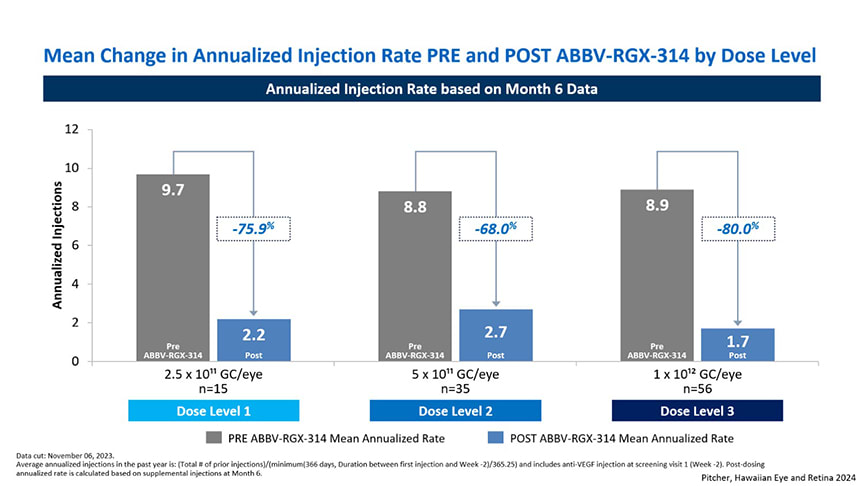
Figure 2. Patients in AAVIATE experienced sharp reduction in their annualized anti-VEGF injection rate across all dose cohorts.
The potential of suprachoroidal delivery lies not only in its efficacy but also in its accessibility compared to surgical subretinal delivery. Still, Dr. Boyer emphasized that transfection efficiency is central to its long-term success.
“If you can get significant transfection in that space, you can treat in that space,” he said. “Obviously, if you look at the results … subretinal delivery is the best as far as the transfection. But suprachoroidal may be the sweet spot—less invasive than subretinal, and more targeted than intravitreal.”
As the field moves toward long-acting or 1-time treatments for chronic retinal diseases, the ability to deliver gene therapy safely and effectively outside of the operating room may help reduce treatment burden and broaden access. “I just want to thank all the patients, investigators, and coordinators who are trying to get us closer to ‘one and done,’” concluded Dr. Boyer. RP

Figure 3. David S. Boyer, MD, outlined the advantages and early results of suprachoroidal gene therapy during the the 2025 Retina World Congress in Fort Lauderdale, Florida.








