A large, retrospective analysis of more than 20,000 eyes treated for geographic atrophy (GA) suggests that several baseline clinical characteristics—including coexisting neovascular age-related macular degeneration (nAMD) and poor initial visual acuity—are significantly associated with early discontinuation of intravitreal complement inhibitor therapy.
Presented by Deepak Sambhara, MD, FASRS, at the 2025 Retina World Congress in Fort Lauderdale, Florida, the findings draw on data from the Vestrum Health database, a national panel of deidentified electronic health records contributed by private retina specialists. The analysis represents an independent, pharmaceutical industry–agnostic project, conceived and executed in collaboration with Vestrum Health, Dr. Sambhara noted. Subjects were included if they had GA secondary to AMD, at least 1 follow-up visit during the study period (February 2023 through March 2025), and documented receipt of an FDA-approved GA therapy. Exclusion criteria removed those with non-AMD diagnoses or incomplete demographic data. Baseline demographics—including visual acuity, presence of subfoveal atrophy, and concurrent wet AMD—were captured to assess correlation with long-term adherence.
The study included 20,671 eyes from 15,002 patients with GA. Of these, 68% of eyes were treated with pegcetacoplan (Syfovre; Apellis Pharmaceuticals) and 32% received avacincaptad pegol (Izervay; Astellas Pharma). The overall cohort had a mean age of 82.1 years, and 67.5% were female. At baseline, 31.6% of study eyes had coexisting neovascular AMD, and 37.7% of fellow eyes had the condition. Anti-VEGF treatment history was common, seen in 26.8% of study eyes and 30.7% of fellow eyes. Mean visual acuity across the cohort was 55 ETDRS letters, and more than half (52.9%) had foveal involvement at baseline.
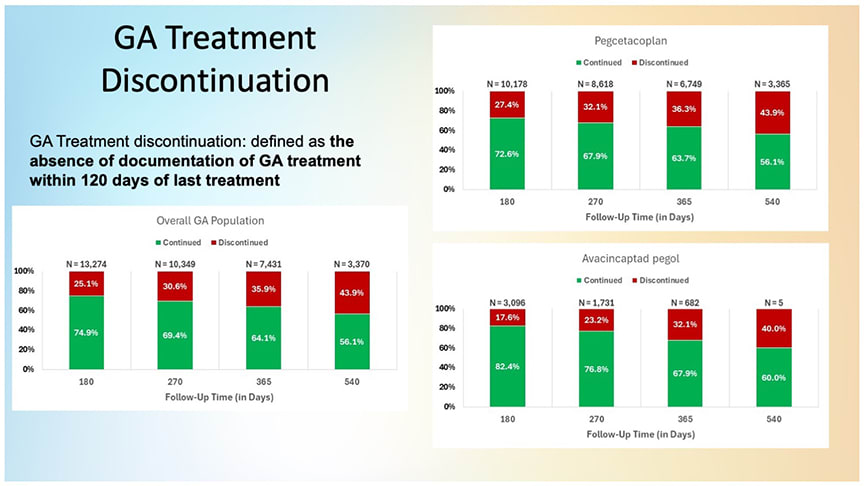
Figure 1. The trend across time points indicates increasing attrition among patients receiving complement inhibition treatments for geographic atrophy (GA). Discontinuation rates increase the longer patients are on therapy, regardless of the treatment agent used.
The researchers found that discontinuation—defined as a treatment gap exceeding 120 days—increased over time regardless of which agent was used (Figure 1). “There is significant attrition that happens over 18 months in patients receiving intravitreal treatments for GA, irrespective of agent,” noted Dr. Sambhara, a consultant for both Apellis and Astellas. “The further out in time you go, the greater levels of attrition you see. That’s not unique to 1 agent vs another, that’s a reality of GA treatments.”
Dr. Sambhara warned against interpreting dropout rates without considering follow-up eligibility and other factors. Differences in FDA approval dates (February 2023 for pegcetacoplan and August 2023 for avacincaptad pegol) complicate direct comparisons between treatment groups, and relatively few patients—particularly those receiving avacincaptad pegol—had reached the 18-month interval, which limits conclusions about long-term persistence.
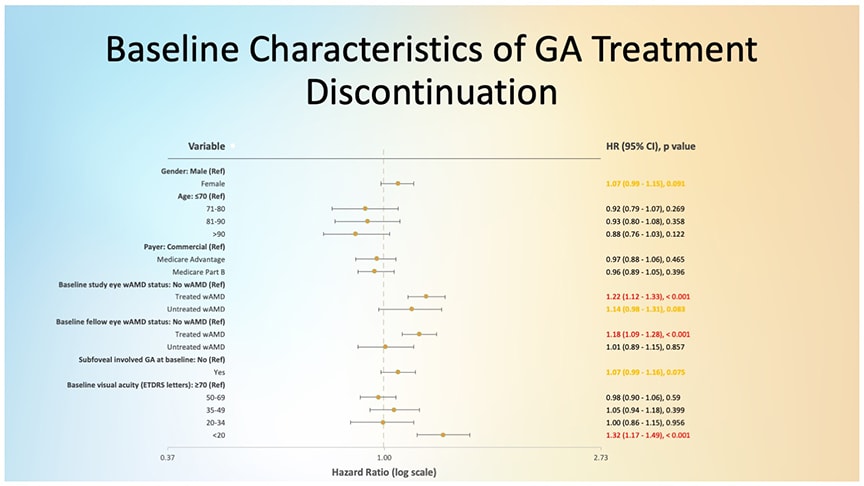
Figure 2. Cox multivariate analysis identified several baseline factors (highlighted in red) that were most strongly associated with discontinuation of GA treatment.
The team applied a Cox multivariate model to isolate baseline characteristics predictive of discontinuation (Figure 2). Among the most significant were as follows:
- Wet AMD in the study eye at baseline. “If you are actively being treated for wet AMD in the same eye that you’re getting shots for GA, that’s actually predictive of discontinuing GA shots,” Dr. Sambhara said.
- Wet AMD in the fellow eye being treated at baseline. Independent of treatment in the study eye, patients actively receiving anti-VEGF injections in the contralateral eye were also more likely to stop GA treatment.
- Poor baseline vision. Eyes presenting with ≤20 ETDRS letters at initiation were more likely to discontinue. “If you’re starting poor, you are more likely to stop doing shots and pull the plug earlier,” he said. “Whether it’s the patient, or you as a physician consulting with the patient, making that decision together.”
The study underscores the challenges retina specialists face in maintaining long-term compliance with GA therapies. Dr. Sambhara noted that further longitudinal analysis will be needed to clarify the specific causes behind discontinuation, whether due to disease progression, adverse events, treatment fatigue, or physician/patient–driven decision-making. “Additional follow-up is required to further elucidate reasons for discontinuation,” he concluded, “to better understand the trends seen in this real-world data set.” RP