A new topical eye drop for diabetic macular edema (DME) may offer an alternative to intravitreal injections, which has long been the standard treatment. In results from the DIAMOND stage 1 trial presented at the 2025 Retina World Congress, OCS-01 (Oculis), a high-concentration (15 mg/mL) dexamethasone formulation, met both its primary and secondary endpoints, showing statistically significant gains in vision and reductions in retinal thickness.
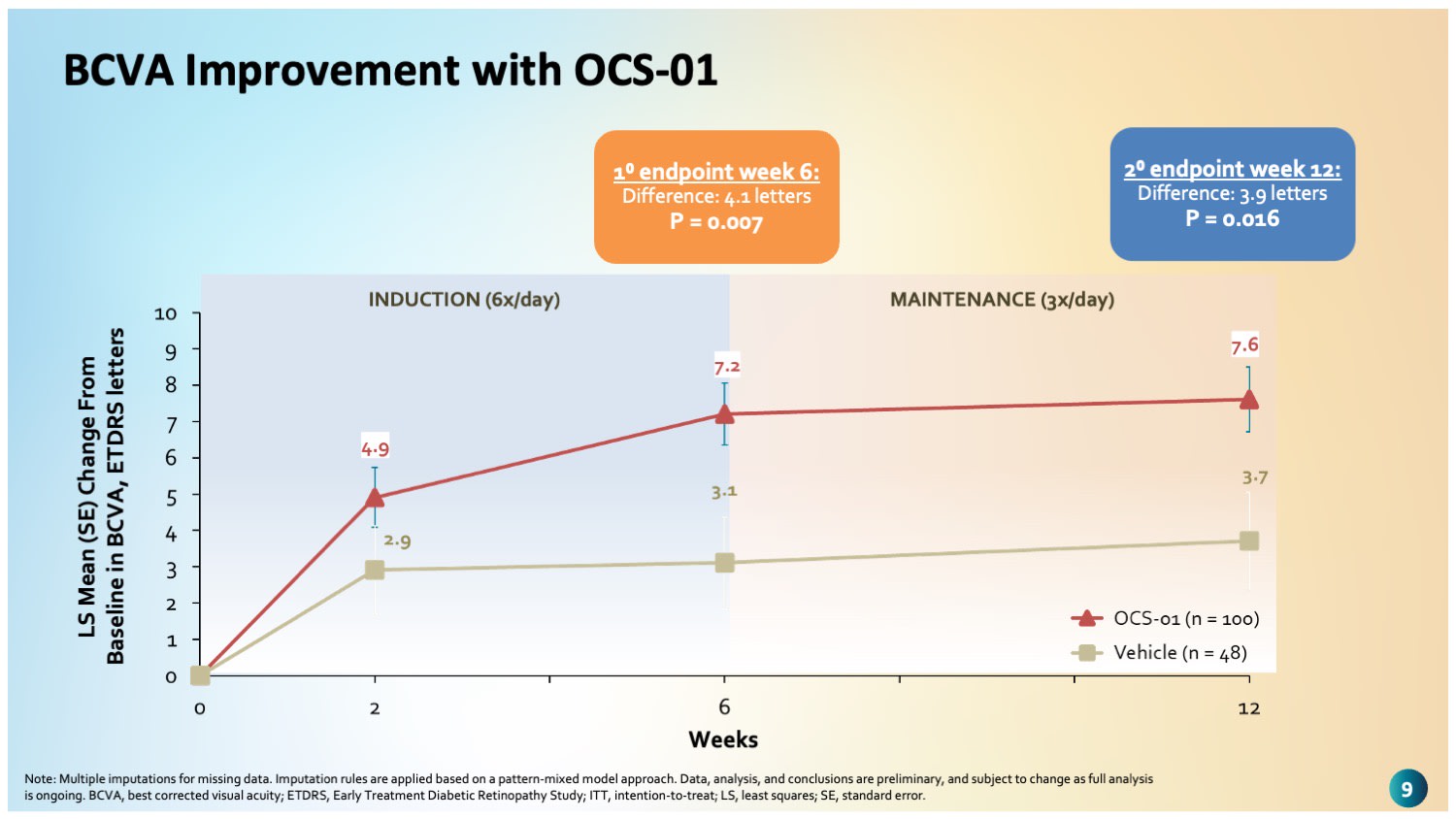
Figure 1. Patients on OCS-01 had rapid and statistically significant improvement in best-corrected visual acuity vs placebo as early as week 2, which was sustained through week 6, the primary endpoint of DIAMOND stage 1.
“We’re hoping this will be the first topical treatment for DME, the number one cause of vision loss in the working population,” said David Almeida, MD, MBA, PhD, president of Erie Retina Research, a DIAMOND investigator and consultant to Oculis. “This is such a big group of patients that a drop, rather than intravitreal injections, would be really welcome.”
DIAMOND is a 2-stage phase 2/3 trial assessing the safety and efficacy of OCS-01 in DME. In stage 1, 148 patients were randomized 2:1 to receive OCS-01 or placebo eye drops 6 times daily during a 6-week loading phase, followed by 3 times daily for 6 weeks in a maintenance phase.
“The primary endpoint was achieved with high statistical significance,” noted Dr. Almeida, citing a mean BCVA improvement of 7.2 EDTRS letters in the OCS-01 group at week 6 compared to 3.1 letters in the placebo arm (P=.007). Gains were evident as early as week 2 and were sustained through week 12, where patients on OCS-01 gained an average of 7.6 letters vs 3.7 in the placebo group (P=.016), he said (Figure 1).
“We saw rapid and statistically significant reductions in retinal thickness beginning at week 2,” Dr. Almeida added. At week 6, CST decreased by 69.1 µm more in the OCS-01 group than in the placebo group. The CST benefit was maintained at week 12, with a between-arm difference of 45 µm (Figure 2).
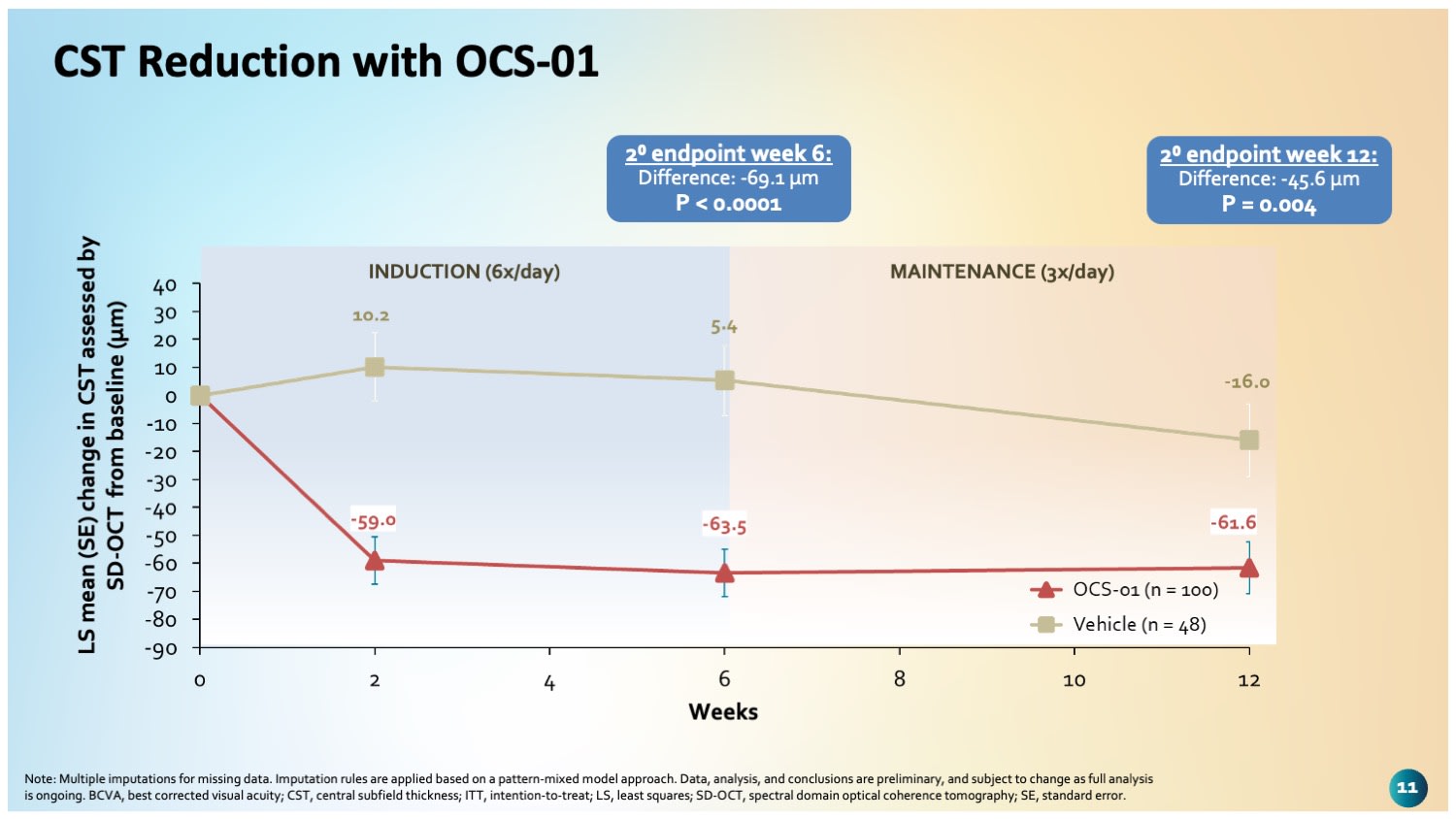
Figure 2. Patients on OCS-01 had statistically significant reductions in central subfield thickness (CST) vs placebo through the maintenance phase, with a difference in CST of 45.6 µm at week 12.
A significantly greater proportion of patients treated with OCS-01 achieved a ≥15-letter BCVA gain at every measured time point. By week 12, 27.4% of patients in the treatment arm achieved this threshold, compared to 7.5% in the control arm (P=.009).
Subgroup analyses by prior treatment status and lens status showed consistent benefits. “Both treatment-naïve and previously treated patients experienced visual and anatomic improvements with OCS-01,” Dr. Almeida said. Treatment-naïve patients gained 7.5 letters vs 2.8 letters with placebo, and previously treated patients gained 6.4 letters vs 3.6 letters. CST reductions in these groups were 71 µm and 52.3 µm, respectively, in favor of OCS-01. Among phakic patients, the letter gain at week 6 was 6.9 with OCS-01 vs 3.0 with placebo. Among pseudophakic patients, the gain was 7.8 letters vs 3.6 letters. CST reductions were 58.7 µm and 91.9 µm, respectively.
Intraocular pressure (IOP) increases were more common with OCS-01. “There were 22 cases of IOP elevation in the treatment group, and 11 of those required medication,” Dr. Almeida noted. “Based on previous studies, IOP tends to return to baseline after discontinuation.” One treatment-emergent adverse effect related to increased IOP in the study eye in the OCS-01 group led to discontinuation of the study drug and withdrawal from DIAMOND, he said.
Dr. Almeida emphasized the unique mechanism of OCS-01, which enables topical delivery of steroid to both anterior and posterior segments of the eye. “This formulation increases solubility and residence time, driving drug into the eye at efficacious concentrations,” he said.
Stage 2 of DIAMOND, with longer follow-up, is currently enrolling. “We’re hopeful that by next year we’ll have data from the phase 3 program to share,” Dr. Almeida said. RP

Figure 3. David Almeida, MD, MBA, PhD, discusses the stage 2 DIAMOND trial of OCS-01 in diabetic macular edema, which is currently enrolling.








