An analysis of Medicare claims for intravitreal anti-VEGF injections indicates that retina specialists often begin treating patients with neovascular age-related macular degeneration (nAMD) using low-cost compounded bevacizumab, then switch many of them to branded anti-VEGF agents within months or years, leading to steep increases in drug-related expenditures. Robin A. Vora, MD, chair of ophthalmology at The Permanente Medical Group (TPMG) in Oakland, California, presented the results of a study on real-world anti-VEGF treatment patterns among Medicare patients at the 2025 Retina World Congress in Fort Lauderdale, Florida.
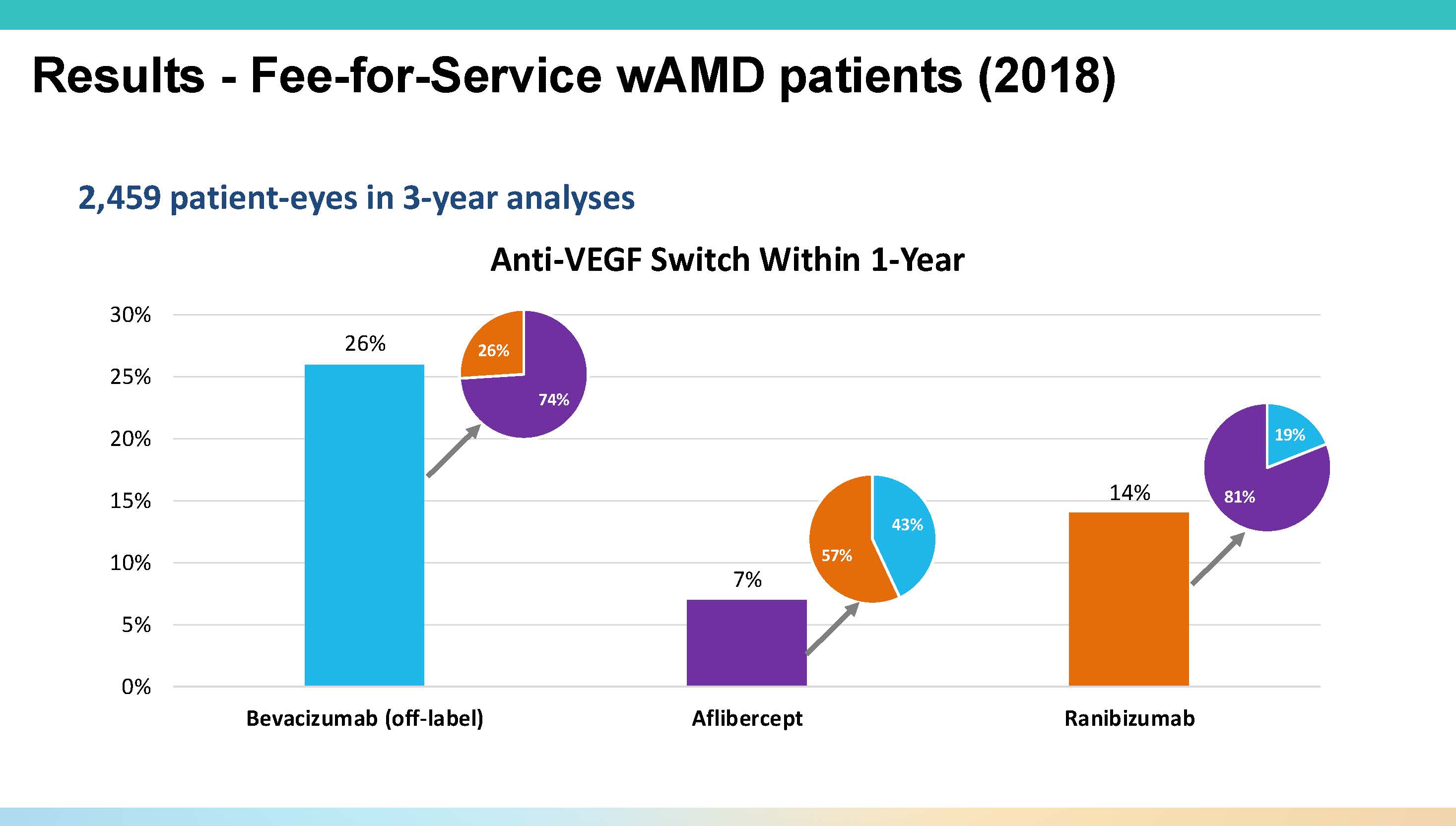
Figure 1. In analysis of Medicare data from 2018 to 2021, 54% of neovascular age-related macular degeneration patients began anti-VEGF therapy with bevacizumab. However, within a year 26% had switched to another agent.
“We wanted to evaluate the impact of start-and-switch decisions on healthcare costs in treatment-naïve wet AMD patients,” explained Dr. Vora. The research followed 2 cohorts—1 of fee-for-service Medicare patients tracked over 3 years, and 1 of Medicare Advantage patients followed for a year. In both groups, most patients began treatment with bevacizumab (Avastin; Genentech), a compounded, off-label anti-VEGF agent.
In the fee-for-service cohort of nearly 2,500 patients who initiated treatment in 2018, more than half (54%) started with bevacizumab, with 41% starting with aflibercept 2 mg (Eylea; Regeneron) and the remainder on ranibizumab (Lucentis; Genentech). “If you follow these patients for a year, about 25% of them come off Avastin and go mostly to aflibercept,” Dr. Vora noted. “But if you start with aflibercept, only 7% come off that drug—so generally, once you’re in the branded network, you stay there” (Figure 1).
Over 3 years, the switch rate from bevacizumab rose to 40%, and bevacizumab starters were significantly (P<.001) more likely to switch at every 6-month timepoint. This switching trend has significant cost implications. “If you were to stay on bevacizumab for 3 years, drug costs are about $700,” Dr. Vora said. “But once you make that switch, on average, the 3-year cost of care for that patient rises to about $20,000” (Figure 2).
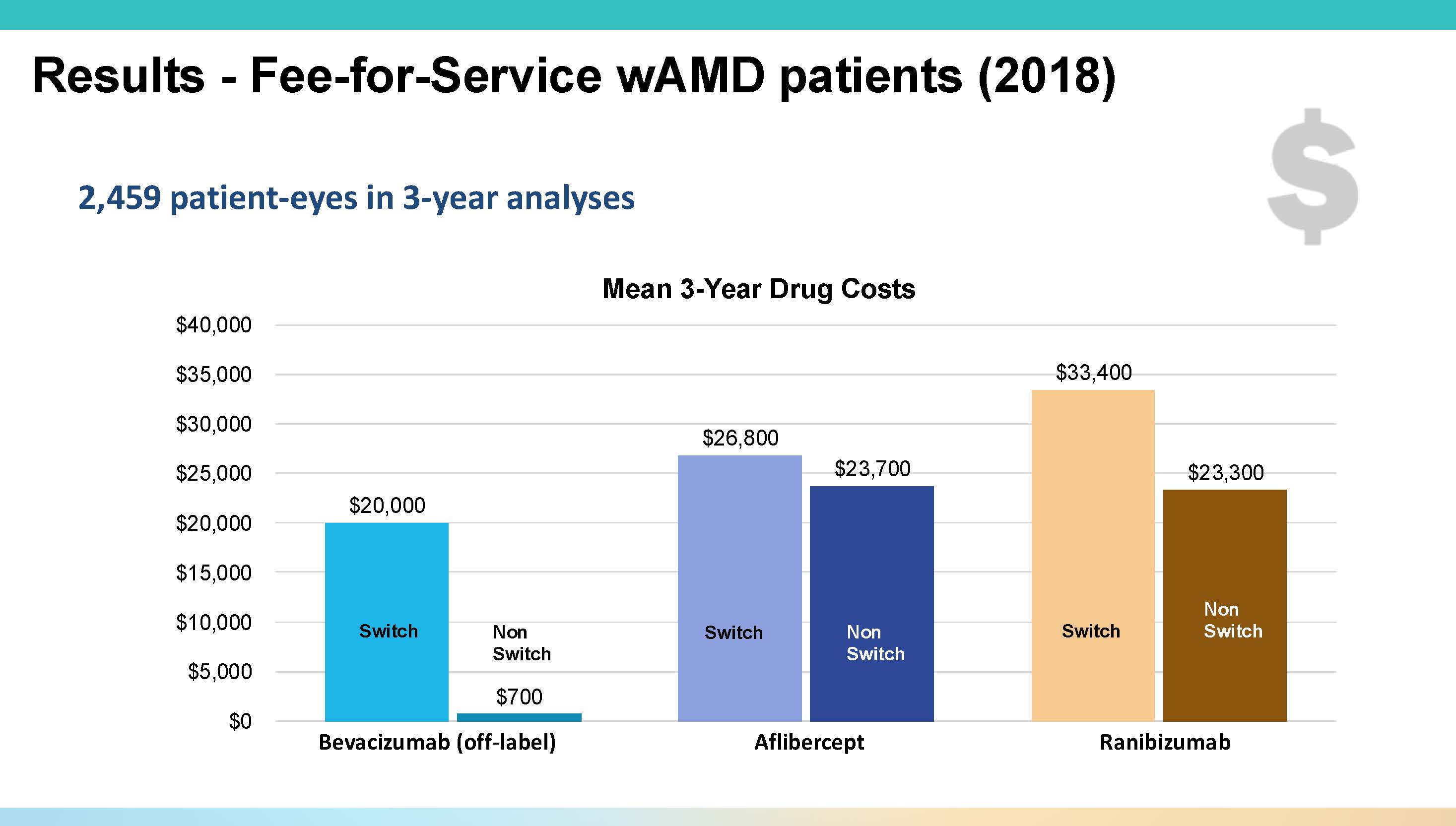
Figure 2. The 3-year average cost of treating with bevacizumab is around $700, compared to roughly $20,000 after switching to another anti-VEGF agent.
In the 1-year Medicare Advantage analysis, step therapy protocols were more common, but the pattern persisted. Seventy percent of patients began treatment with bevacizumab, and more than half of those patients switched, mostly to aflibercept. “By 2022, you start to see faricimab (Vabysmo; Genentech) entering the picture,” Dr. Vora noted. “About one-quarter of the aflibercept patients were now moving over to faricimab, which I think also reflects what we’re seeing in clinical practice" (Figure 3).
Again in this second analysis, the potential for cost savings by staying on bevacizumab was striking, but real-world data showed that such practice was rare. “Instead of spending $500 a year, you’re now spending $7,500,” he added, referring to undertreated patients who switch to branded drugs after inadequate response.
Dr. Vora suggested that the high attrition rate from bevacizumab might be partially attributable to the compounded drug’s variable quality. In a study from 2015, he said, researchers ordered 22 samples from 11 pharmacies, and 81% of those samples had reduced protein levels compared to what you’d get from a standard manufacturing source.1

Figure 3. High switch rates from bevacizumab, seen in the study of Medicare Advantage patients, may be due to concern about supply problems or issues with compounding.
Even within a single pharmacy, 30% of syringes had inconsistent protein concentrations. “Because compounding requires human effort—to draw a large vial into small syringes—and that is error prone,” Dr. Vora said. “That can lead to underdosing and undertherapeutic treatment, which unfortunately ends up being costly.”
Dr. Vora concluded by urging further investigation into the reasons behind the frequent switching. “Is it a molecule issue? Or is it a compounding and supply issue?” he asked. An approved, ophthalmic formulation of bevacizumab could help answer these questions by ensuring consistent potency and supply, potentially reducing the need to switch patients, he said.
The US Food and Drug Administration (FDA) is currently evaluating a Biologics License Application for ONS-5010 (Outlook Therapeutics), an investigational ophthalmic formulation of bevacizumab that has already been approved in the European Union and the United Kingdom. The FDA has set a PDUFA date of August 27, 2025.
“Hopefully … we may have this answer in August,” concluded Dr. Vora, a consultant to Outlook Therapeutics. RP

Figure 4. Robin A. Vora, MD, disucsses the impact start-and-switch decisions on healthcare costs at the Retina World Congress in Fort Lauderdale, Florida.
Reference
1. Yannuzzi NA, Klufas MA, Quach L, et al. Evaluation of compounded bevacizumab prepared for intravitreal injection. JAMA Ophthalmol. 2015;133(1):32-39. doi:10.1001/jamaophthalmol.2014.3591








