Radiation retinopathy remains the leading cause of vision loss following plaque brachytherapy for choroidal melanoma. However, emerging research is changing the ways that ophthalmologists manage, or potentially prevent, this complication, according to Kapil Mishra, MD, who presented at the 2025 Retina World Congress in Fort Lauderdale, Florida.
Vision loss doesn’t occur immediately after melanoma treatment, explained Dr. Mishra, a clinical assistant professor of ophthalmology, vitreoretinal surgery, and ocular oncology at the Gavin Herbert Eye Institute of the University of California, Irvine. Instead, it typically appears 6 months to 3 years later, with features that are clinically indistinguishable from diabetic retinopathy (Figure 1). Radiation retinopathy can be difficult to manage once established.
“The macular edema can be pretty resistant to treatment,” Dr. Mishra noted, adding that half of patients had vision of 20/200 or worse at 3 years in early studies. Current treatment strategies largely mirror those for diabetic retinopathy, including anti-VEGF injections and panretinal photocoagulation, but researchers are now also focusing on preventing retinopathy altogether, Dr. Mishra explained.
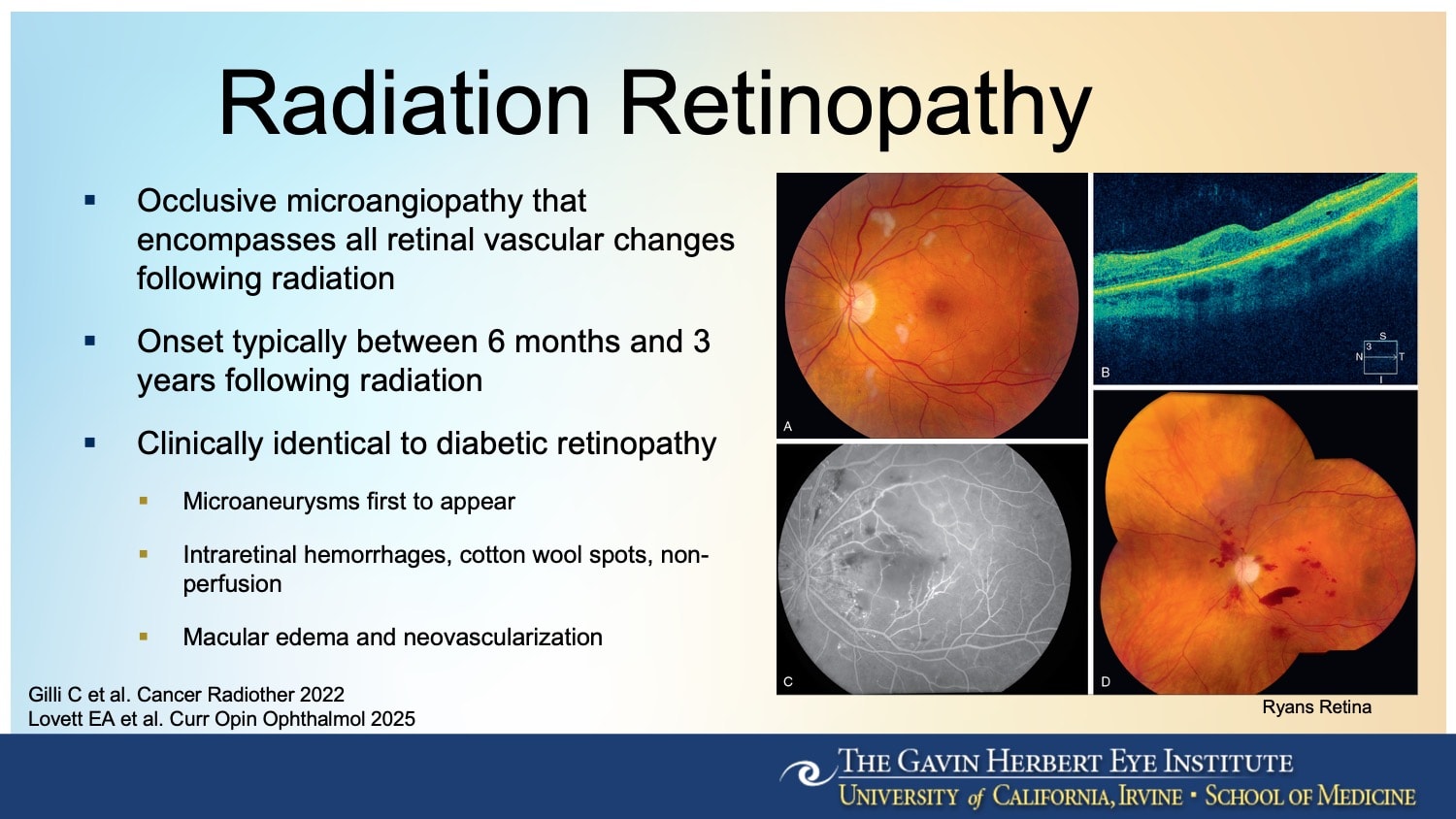
Figure 1. Radiation retinopathy presents with clinical features that are indistinguishable from diabetic retinopathy, including intraretinal hemorrhages, cotton wool spots, macular edema, and vascular nonperfusion.
Reducing Radiation
Among the most direct interventions is reducing the radiation dose itself. Typically, these tumors are treated with an 85 Gy radiation dose at the apex, the standard set by the Collaborative Ocular Melanoma Study. This dose provides effective tumor control but can damage surrounding retinal tissue. “If you treat with less radiation, then there’s lower chance of radiation retinopathy, or it is less severe,” explained Dr. Mishra. “Some centers are looking to treat at a lower dose of 63 Gy to reduce the severity of the retinopathy.”1
Technology also plays a role, he noted. “Historically, we used standard-size plaques, which could lead to overtreatment,” he said. “Now companies can design plaques that are custom-made for the patient, with the goal of reducing radiation exposure to the retina.”
In some cases, it may be possible to avoid radiation altogether. Thanks to a better understanding of tumor genetics, oncologists can now identify patients whose tumors have low metastatic potential. “If you treated fewer patients with radiation for the tumors that have a very low risk of metastasizing, then maybe you reduce the overall incidence of radiation retinopathy,” said Dr. Mishra. “If you biopsy and find a low-risk mutation, you may defer radiation or choose a different approach.”
New Treatments in Trials
A pair of investigational systemic therapies are also drawing attention. The first is darovasertib (Ideaya Biosciences), a protein kinase C inhibitor being studied as a neoadjuvant treatment for uveal melanoma.2 “Darovasertib has been shown in early studies to shrink the tumor,” Dr. Mishra said. “So, when you do treat with radiation, you can use less radiation because the tumor is smaller.” A phase 2 trial of darovasertib is currently under way, with results expected later this year.
The second systemic therapy is belzupacap sarotalocan, or bel-sar (AU-011; Aura Biosciences), a light-activated molecule.3 Bel-sar is injected into the suprachoroidal space where it binds to tumor cells. It is then activated by a laser in a clinical setting to destroy the tumor without the use of radiation. The treatment is currently being studied in the phase 3 CoMpass trial for small choroidal melanomas and indeterminate lesions.
For patients who have already received radiation, prophylactic intravitreal treatment may help delay or prevent retinopathy altogether. “There’s been some attention to prophylactically treating with anti-VEGF before the clinical onset of retinopathy,” Dr. Mishra said. Retrospective studies suggest that this approach can reduce the incidence and severity of the condition (Figure 2).4
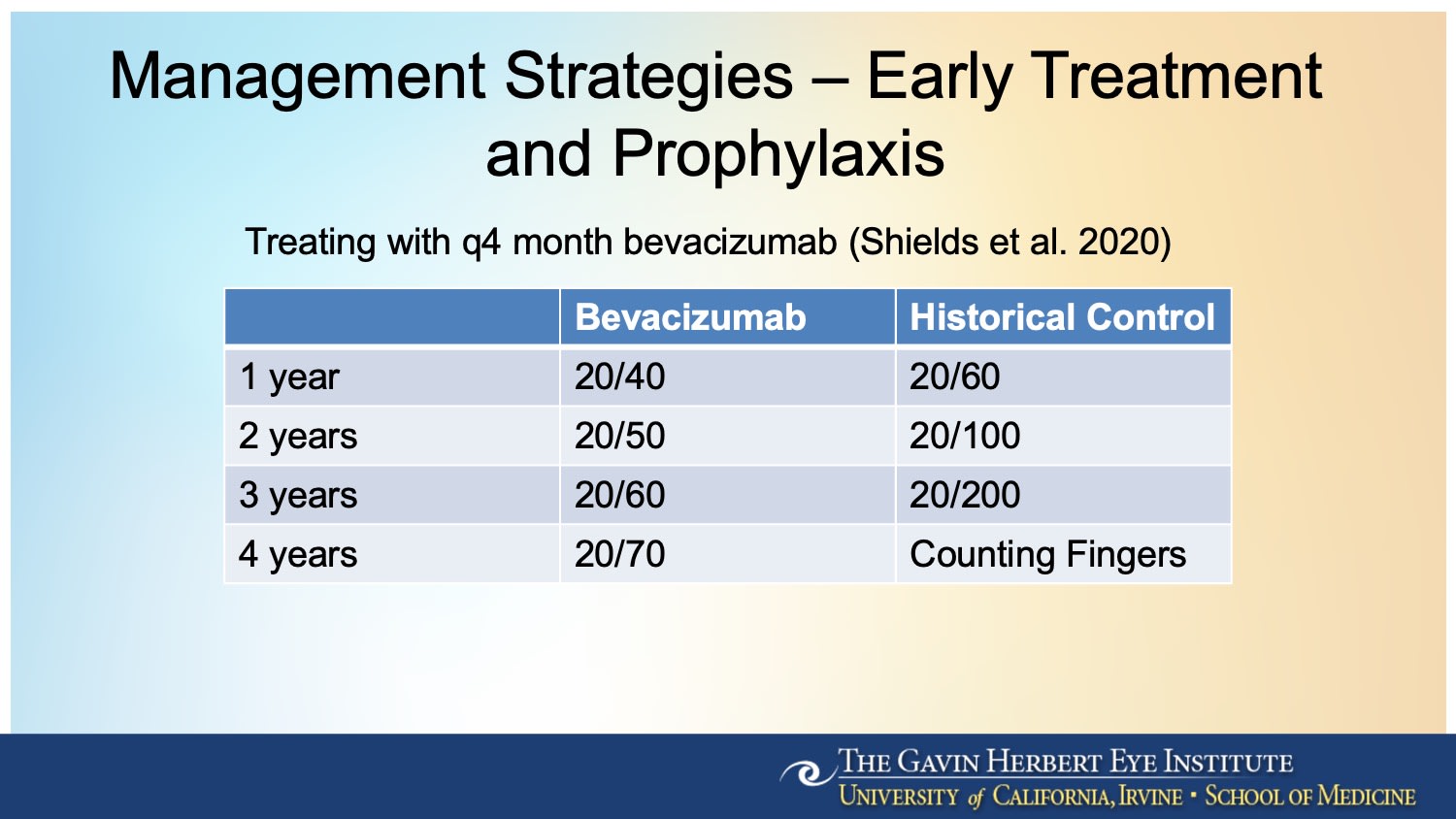
Figure 2. In a cohort study of 1,131 eyes with plaque-irradiated uveal melanoma treated with intravitreal bevacizumab (Avastin; Genentech) at 4-month intervals for 2 years, visual outcome was better at 1, 2, 3, and 4 years, with 4-year median visual acuity of 20/70 compared with counting fingers in a nonrandomized control group.3
A randomized clinical trial is currently under way to study prophylactic treatment. The DRCR Retina Network’s Protocol AL compares intravitreal injections of faricimab 6 mg (Vabysmo; Genentech) and fluocinolone acetonide 0.19 mg intravitreal implants (Iluvien; Alimera Sciences) to observation without immediate treatment. The trial aims to determine whether early intervention with anti-VEGF therapy or sustained-release corticosteroids can prevent vision loss due to radiation retinopathy in patients undergoing plaque brachytherapy for primary choroidal melanoma.
“This is the first randomized controlled trial looking at these options,” Dr. Mishra said. “It’s very exciting to have the DRCR network conduct an ocular oncology trial.”
Taken together, these approaches—reducing the radiation dose, genetic risk stratification, systemic therapy, and prophylactic treatment following radiotherapy—may shift the paradigm for how radiation retinopathy is managed. “These are some of the most promising avenues we have right now,” Dr. Mishra said. “And they’re all focused on preserving vision without compromising tumor control.” RP
References
1. Kheir WJ, Stinnett SS, Meltsner S, et al. Preliminary results of uveal melanoma treated with iodine-125 plaques: analysis of disease control and visual outcomes with 63 gy to the target volume. Adv Radiat Oncol. 2022;7(2):100869. doi:10.1016/j.adro.2021.100869
2. (Neo)Adjuvant IDE196 (darovasertib) in patients with localized ocular melanoma. Clinicaltrials.gov identifier: NCT05907954. Updated October 28, 2024. Accessed May 9, 2025. https://www.clinicaltrials.gov/study/NCT05907954
3. A phase 3 randomized, masked, controlled trial to evaluate efficacy and safety of belzupacap sarotalocan (AU-011) treatment compared to sham control in subjects with primary indeterminate lesions or small choroidal melanoma (CoMpass). ClinicalTrials.gov identifier: NCT06007690. Updated April 17, 2025. Accessed May 9, 2025. https://www.clinicaltrials.gov/study/NCT06007690
4. Shields CL, Dalvin LA, Chang M, et al. Visual outcome at 4 years following plaque radiotherapy and prophylactic intravitreal bevacizumab (every 4 months for 2 years) for uveal melanoma: comparison with nonrandomized historical control individuals. JAMA Ophthalmol. 2020;138(2):136-146. doi:10.1001/jamaophthalmol.2019.5132








