A phase 1/2a clinical trial evaluating OpRegen (RG6501) allogeneic retinal pigment epithelial (RPE) cell therapy has shown promising 36-month outcomes in patients with geographic atrophy (GA). New data presented last week reveal that treated eyes with extensive subretinal lesion coverage achieved an average gain of +9 ETDRS letters, with accompanying sustained structural improvements, after a single administration.
“These cells are showing incredible promise to restore and recover vision in dry age-related macular degeneration (AMD) patients with GA,” said Christopher D. Riemann, MD, a principal investigator for the trial, who presented the data at the 2025 Clinical Trials at the Summit meeting in Las Vegas. “Other currently approved treatments have good and clinically relevant data showing a reduction in the speed of geographic atrophy growth, but that does not translate into a visual acuity difference in treated vs untreated patients. This is the first time we’ve ever had a conversation about a visual acuity signal that shows improvement.”

Figure 1. OpRegen is an investigational cell therapy that involves transplanting retinal pigment epithelial (RPE) cells derived from human embryonic stem cells to treat geographic atrophy. Image courtesy Genentech.
OpRegen cell therapy (Figure 1) is being developed under an exclusive worldwide collaboration between Lineage Cell Therapeutics, Roche, and Genentech, explained Dr. Riemann, a vitreoretinal surgeon and fellowship director at the Cincinnati Eye Institute and University of Cincinnati School of Medicine. The phase 1/2a study is an open-label, single-arm, multicenter, dose-escalation trial evaluating a single subretinal administration of OpRegen cell therapy in patients with bilateral GA secondary to AMD. Twenty-four patients were enrolled into 4 cohorts. The first 3 cohorts enrolled only legally blind patients with a best-corrected visual acuity (BCVA) of 20/200 or worse. The fourth cohort enrolled 12 patients with impaired vision (BCVA from 20/64 to 20/250) and smaller mean GA lesion areas. Cohort 4 also included patients treated with a new “thaw-and-inject” formulation of OpRegen cell therapy, which can be shipped directly to sites and used immediately upon thawing, removing the complications and logistics of having to use a dose-preparation facility.
In cohort 4, the study eyes showed sustained BCVA gains at 12 months, 24 months, and 36 months, with average gains from baseline of +7.7, +5.5, and +6 EDRTS letters at those respective time points (Figure 2). The untreated fellow eye, meanwhile, was relatively stable for the first 2 years (+1.3 EDRTS letters from baseline at 12 months and -0.8 EDTRS letters at 24 months), but dropped off significantly at year 3 (-7.1 EDTRS letters). “Look at what happens going forward to 3 years—the improvement in OpRegen treated eyes persists, while the fellow eye goes down,” said Dr. Riemann. “That’s just ridiculously powerful data. Plus 6, that’s a full ETDRS line.”

Figure 2. Patients with less advanced geographic atrophy (GA) treated with OpRegen showed a sustained improvement in best-corrected visual acuity (BCVA) at 12, 24, and 36 months. The BCVA gains were greater in the eyes where the cell therapy more extensively covered the area of GA. Image courtesy Genentech.
The gains were most significant in eyes that received complete coverage of the subfoveal GA area, he noted. “Early on in the phase 1 trial, the surgeons were instructed to just inject the OpRegen cells into the macula and hopefully there will be some trophic effects, and that’s what the surgeons did,” explained Dr. Riemann. As a result, 7 eyes in cohort 4 received more limited cell coverage of the GA area under the subretinal bleb. BCVA improvement can be seen in those patients, but the effect is not as pronounced as it is in the 5 patients from cohort 4 whose cell treatment covered most or all of the area of GA. Patients with limited coverage of the fovea under the subretinal bleb showed BCVA gains of 3.9, 3.6, and 3.4 EDTRS letters at 12, 24, and 36 months, compared to gains of 12.8, 7.4, and 9.0 at the same time points in the patients with extensive coverage (Figure 2). “Better OpRegen cell coverage of the GA lesion correlated to better visual acuity. That may be interpreted as a dose response curve of sorts, which is very exciting.”
“These results suggest that we may need these cells to encompass the area of geographic atrophy,” said Dr. Riemann. “We don’t want to be dilly dallying at the edge of the macula—our goal is to get these cells under the macula and to go where the geographic atrophy is and replace the cells that have been lost.”
A second, more objective dose dependent signal was detected by a masked quantitative analysis of optical coherence tomography (OCT) imaging. Sustained structural improvements in retinal layers persisted out to 36 months. Treated eyes with extensive GA coverage demonstrated consistent gains in the external limiting membrane (ELM) and RPE-drusen complex (RPEDC) layers (Figure 3). From 24 to 36 months, mean RPEDC area in treated eyes decreased slightly from +2.6 mm² to +1.9 mm², while fellow untreated eyes declined from -2.8 mm² to -3.8 mm². Similarly, ELM area gains in treated eyes held steady (+0.8 mm² to +0.3 mm²), in contrast to continued deterioration in fellow eyes (-1.9 mm² to -3.4 mm²). “The OCT shows a structural effect that correlates to the functional effect,” noted Dr. Riemann. “If you look at extensive bleb coverage vs limited, you see the greater structural recovery in patients with extensive bleb coverage. They get better RPE drusen complex because you’re putting RPE cells in. In my mind this indicates a powerful dose response.”
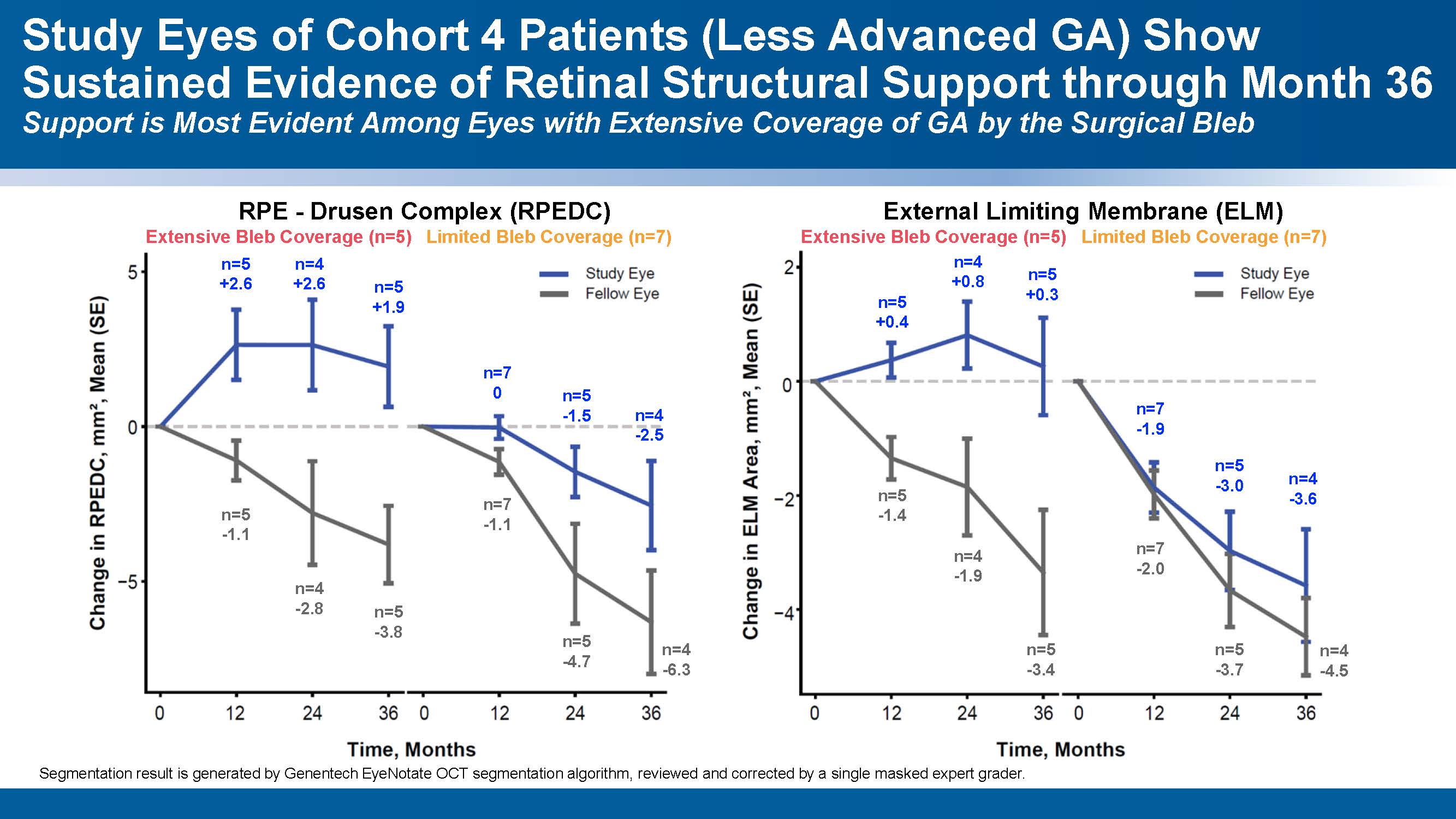
Figure 2. Patients with less advanced GA treated with OpRegen showed evidence of improved retinal structures on optical coherence tomography (OCT) at 36 months. As with the BCVA gains, the improvement was greatest in eyes that received more extensive coverage of the GA area. Image courtesy Genentech.
Safety outcomes included epiretinal membrane formation—presumably from cell extrusion—associated with OpRegen delivery via the vitrectomy/retinotomy approach and CNVM formation when OpRegen cell therapy was administered with the Orbit SDS device. However, Dr. Riemann said, “The good news is you could peel those membranes and treat the CNVM’s with anti VEGF drugs and those patients did well.” Adverse events and unexpected complications were uncommon and manageable over 3 years, he said.
Identifying the optimal way to surgically deliver the OpRegen cell treatment is a goal of the phase 2a GAlette study, which is currently underway, said Dr. Riemann. The open-label, multicenter clinical trial is currently enrolling up to 60 patients across multiple sites in the United States and Israel, with participants receiving a single subretinal administration of OpRegen cell therapy in 1 eye. Three delivery methods will be evaluated: standard subretinal vitrectomy/retinotomy techniques, a next-generation Orbit SDS device, and a proprietary transvitreal subretinal injector with a dual lumen. Key study endpoints include safety and tolerability, anatomical changes observed by OCT, and functional outcomes such as changes in BCVA. The study also aims to further clarify the relationship between surgical technique, bleb coverage of the atrophic lesion, and long-term treatment response.
Dr. Riemann stressed measured expectations: “This is buzzworthy, but we’re talking about small numbers—it’s 3-year data on 12 patients. That does not equate to, ‘We’ve got a cure!’ How many phase 1 treatments have looked amazing and then failed in phase 2 or 3. But this is really cool, really exciting data and I can’t wait to see where it goes next.” RP








