Time is retina! A central retinal artery occlusion (CRAO) is a stroke of the eye. This ophthalmic emergency occurs at an incidence of approximately 1.9/100,000 in the United States, presenting with acute painless, monocular vision loss.1 Diagnostic findings include severe vision loss, relative afferent pupillary defect, and funduscopic examination revealing retinal hypoperfusion with a characteristic “cherry-red spot” in the macula.2
Rapid diagnosis and treatment of CRAO is necessary to restore vision and manage risk factors that may lead to other vascular ischemic end-organ disease, such as cerebral stroke.3
Remote diagnosis of CRAO can reduce the time to treatment for eligible patients and therefore increase the chance of visual recovery. A novel clinical diagnostic protocol using a remote consult model aims to streamline identification of a CRAO at the point of care.
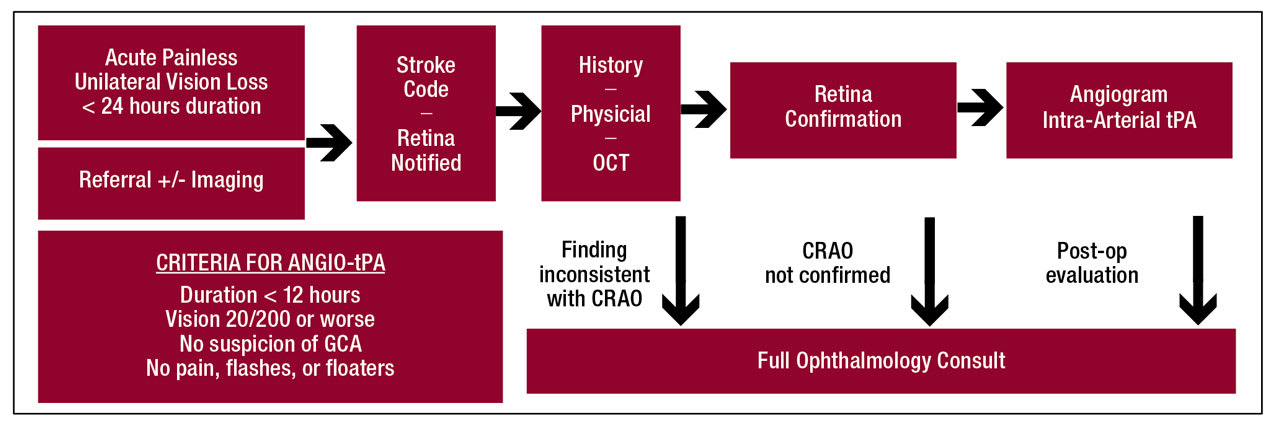
Figure 1. Algorithm for remote retinal artery occlusion diagnosis and criteria for angiogram and intra-arterial tissue plasminogen activator treatment in patients presenting with acute painless unilateral vision loss for less than 24 hours. ANGIO-tPA = angiogram and intra-arterial tissue plasminogen activator; CRAO = central retinal artery occlusion; GCA = giant cell arteritis.
Protocol Design
The eye stroke protocol (ESP) was designed to identify a retinal artery occlusion in patients who present to the emergency department (ED) with painless monocular vision loss, but patients can also be referred directly for intervention. The ESP is fully remote and does not require an in-person consult; however, in-house ophthalmologists can aid in diagnosis when available.4
When patients present to the ED, a stroke code is activated by the emergency medical staff per the stroke center’s protocol (Figure 1). The stroke neurology service would then evaluate the patient, perform a focused history and physical exam, alert the ophthalmology service, and acquire macular optical coherence tomography (OCT) scans of both eyes. The key diagnostic findings include inner retinal hyperreflectivity, thickening of the inner retina, and loss of distinction between its layers (Figure 2). A characteristic “foveal glow” is analogous to a cherry-red spot in fundus photos. The reflectance signal is preserved through the fovea but blocked through the outer retina adjacent to the fovea, creating a spotlight effect.
The images can be transferred directly to the ophthalmology team to identify retinal ischemia. If the diagnosis of a CRAO is confirmed, the patient can be treated immediately if eligible. Treatment can be either with intravenous tissue plasminogen activator (tPA) if the patient is within 4.5 hours of last known well (LKW), or cerebral angiography can be done with intra-arterial injection of tPA directly into the ophthalmic artery. Intra-arterial tPA is given in 2 mg increments every 5 minutes until visual improvement, restoration of choroidal blush on angiogram, or a maximum dose of 22 mg. All patients, whether treated or not, are admitted for a stroke evaluation.
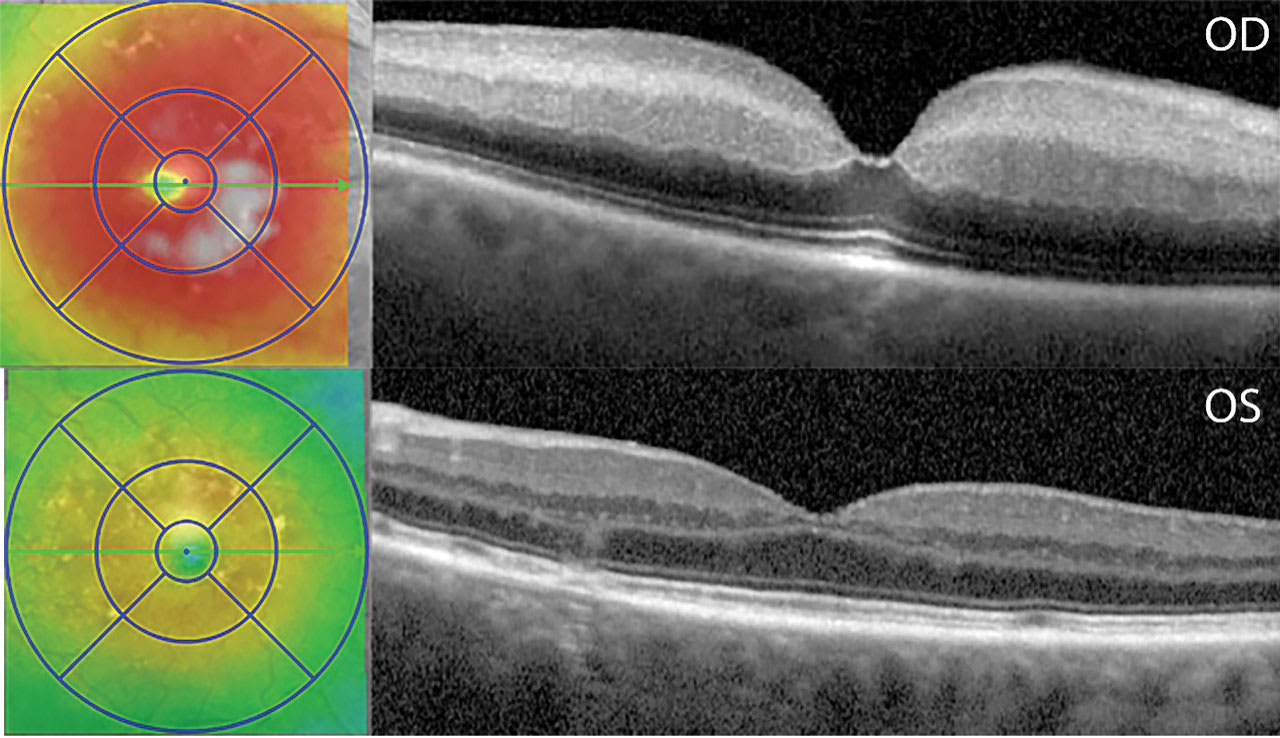
Figure 2. Macular optical coherence tomography (OCT) scan of acute central retinal artery occlusion of the right eye (OD; top) compared to the unaffected contralateral eye (OS; bottom), OCT findings of the right eye show thickening, inner retinal hyperreflectivity, loss of differentiation of the inner retinal layers, inner retinal thickening, decreased signal transmission to the outer retinal layers and choroid, and the “foveal glow.”
Unique Protocol Considerations
A few key features had to be considered during protocol development, and these are applicable to remote consult initiatives in general. First, buy-in from all services is required. This protocol involves collaboration across the ophthalmology service, the ED, and the stroke service. This requires a balance between additional effort and utility. For instance, the protocol uses lines of communication that are parallel to those already used by the stroke team. This minimizes changes to the stroke team’s workflow. The increased effort to acquire OCT images is balanced by the value of more efficient access to ophthalmology diagnostic expertise.
Second, the OCT machines should be placed in convenient and quickly accessible locations that are unique to each stroke center. These locations include next to the CT scanner in radiology, in an exam room in the stroke center adjacent to the ED, and in an ophthalmology room in the ED. Third, the OCT must be user friendly, even for inexperienced operators.
Fourth, the protocol eliminates unnecessary diagnostic testing. Ultrasound can help identify a vitreous hemorrhage or retinal detachment. But these would cause the OCT to fail, prompting a full consult or transfer to a facility where that could be done. Fundus photography would obviously add value, but at the time this protocol went live, devices that acquired fundus photos were more complicated to use.
Finally, establish a communication system with redundancy so that retina surgeons will be immediately accessible but not overburdened. Alerts that there may be a CRAO are sent to 4 physicians simultaneously. If no one responds within a few minutes, the retina call service is notified to reach one of the protocol designers (so far, this has not happened). The backup exists because busy clinicians will have conflicts. Retina surgeons cannot stop a case to read an OCT image, for example, so redundancy is needed in the system.
In summary, logistics matter, and they must be tailored to the existing idiosyncrasies and policies of the health system to increase the likelihood of success.
Initial Protocol Outcomes
During the first 18 months of implementation of the protocol, a total of 59 patients were evaluated, with 42% confirmed CRAOs. Of the 10 patients who met treatment criteria (40%), 9 patients received treatment with IA-tPA (mean dose of 16 mg) within an average of 9 hours after their LKW.
Visual acuity improved significantly within 24 hours of treatment, from a baseline of logMAR 2.14 (counting fingers to hand motions) to 0.70 (20/100). Notably, 66% of patients improved from worse than 20/200 to 20/100 or better. In addition, 44% of treated patients improved to 20/40 or better within 24 hours of treatment. At 1 month, vision remained stable in all but 1 patient who experienced reocclusion and persistent vision loss. No intracranial hemorrhages or systemic complications were observed.4
After implementation of the protocol, the time from door to treatment improved by just over 2 hours for patients who presented to the ED with painless monocular vision loss.
Conclusions and Future Directions
Retinal artery occlusions can be managed through a collaborative model that integrates remote consultation with point-of-care automated OCT, enabling rapid diagnoses and timely administration of tPA when indicated. In an initial study, this approach was associated with improved visual outcomes with treatment. Continued work is needed to validate these findings and define optimal diagnostic and management algorithms for CRAO and other ophthalmic emergencies. RP
References
1. Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820-823.e2. doi:10.1016/j.ajo.2011.05.005
2. Mehta N, Marco RD, Goldhardt R, Modi Y. Central retinal artery occlusion: acute management and treatment. Curr Ophthalmol Rep. 2017;5(2):149-159. doi:10.1007/s40135-017-0135-2
3. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15(1):63-77. doi:10.1007/s11940-012-0202-9
4. Lema GMC, Leacy RD, Fara MG, et al. A remote consult retinal artery occlusion diagnostic protocol. Ophthalmology. 2024;131(6):724-730. doi:10.1016/j.ophtha.2023.11.031










