Earlier this year, EyePoint Pharmaceuticals released positive phase 2 data from VERONA, a clinical trial evaluating the efficacy and safety of the vorolanib intravitreal insert (EYP-1901) in diabetic macular edema (DME). To explore the study results and the EYP-1901 clinical trial programs, I spoke with Ashkan M. Abbey, MD, a principal site investigator for VERONA, and Jay S. Duker, MD, the CEO of EyePoint Pharmaceuticals. I also sought the perspective of Talia R. Kaden, MD, who did not participate in the EYP-1901 clinical trial program. Our conversation has been edited for length and clarity.
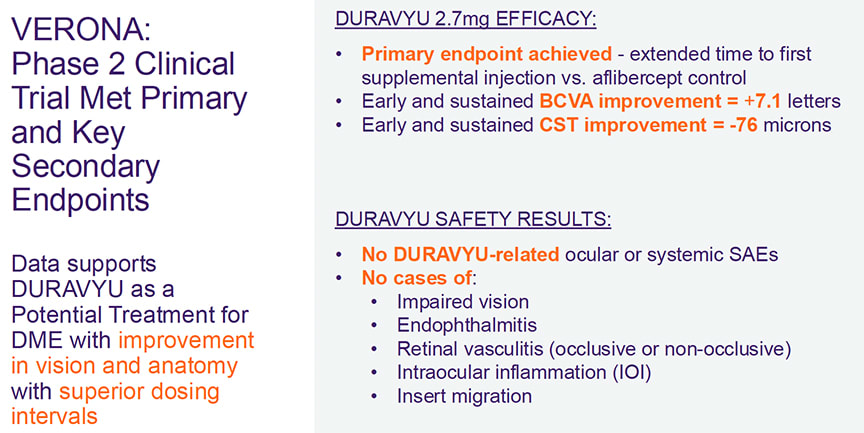
Figure 1. EYP-1901 (also called Duravyu) met its primary endpoint in VERONA, with both vorolanib doses demonstrating extended time to first supplemental treatment vs aflibercept.
Dr. Modi: Dr. Abbey, you have been involved in the vorolanib intravitreal insert clinical program for years. Can you describe the design of the phase 2 study and the rescue criteria? It’s a bit unusual to have 1 injection of aflibercept (Eylea; Regeneron) and then follow patients on a pro re nata (PRN) basis. What was the rationale when designing this study? What do the results suggest when thinking about the durability of vorolanib in DME?
Dr. Abbey: VERONA evaluated the safety and efficacy of the vorolanib intravitreal insert in DME, with a primary endpoint of time to first supplemental anti-VEGF injection vs the aflibercept control arm. All patients received a single aflibercept 2 mg injection at Day 1, followed by either vorolanib 1.3 mg, 2.7 mg, or a sham injection. Patients were followed monthly through 24 weeks and received supplemental anti-VEGF injections per prespecified criteria. Starting at Week 4, criteria were best-corrected visual acuity (BCVA) reduction of 5 to 9 ETDRS letters and CST increase >75 µm on 2 consecutive visits, or BCVA reduction ≥10 letters due to DME, or CST increase ≥100 µm vs baseline. Patients could also receive supplemental injections per investigator discretion. Starting at Week 12, a lack of 10% reduction in CST compared with baseline was also added as a criterium.
As part of the efficacy analyses, VERONA was designed to assess EYP-1901 durability in patients with previously treated DME against a known comparator, in this case, aflibercept. This is why patients received only a single dose at the start of the trial. However, each patient’s welfare was top priority, and patients were evaluated each month for safety and the supplemental treatment per the criteria described above.
EYP-1901 met its primary endpoint in VERONA, with both vorolanib doses demonstrating extended time to first supplemental treatment vs aflibercept. Up to Week 24, 73% of vorolanib 2.7 mg arm patients remained supplement free. Following a single injection of EYP-1901, a clinically meaningful improvement in BCVA with vision gains around 7 letters was reported at Week 24. These vision gains were accompanied by improved and controlled anatomy with a decrease of more than 75 µm in the vorolanib 2.7 mg arm (Figure 1).
Dr. Modi: What was surprising to me was that in the initial 2 months after combination EYP-1901 and aflibercept were administered, there was a visual acuity benefit relative to that of aflibercept. This difference waned over time but what is the proposed rationale to explain this finding?
Dr. Abbey: The early improvements in both BCVA and CST were observed at Week 4, which is the first visit after treatment. This suggests immediate bioavailability of vorolanib from the insert. In the vorolanib 2.7 mg arm, there was a single outlier patient who missed multiple visits, which resulted in a vision loss of >20 letters at the Week 24 visit. When this patient is removed from the analysis, the BCVA change from baseline at Week 24 is +10.1 letters compared with +7.3 letters in the aflibercept arm. Additionally, up to Week 24, 73% of patients in the vorolanib 2.7 mg arm remained supplement free compared with 50% of patients in the aflibercept arm.
Tyrosine kinase inhibitors (TKIs), such as vorolanib, have a different mechanism of action (MOA) compared with typical anti-VEGF therapies. Unlike anti-VEGF therapies, which work extracellularly to block the ligand VEGF-A, TKIs function intracellularly to inhibit all VEGF receptors. This MOA may complement that of typical anti-VEGF therapies and provides rationale for a potential combination approach.
Dr. Modi: As the CEO of EyePoint, can you give us the 30,000-foot picture on EYP-1901, Dr. Duker? What is going on with clinical trial programs, recruitment efforts in the phase 3 trials, and expected read out time?
Dr. Duker: Late last year we initiated our phase 3 trials for neovascular age-related macular degeneration (nAMD), LUCIA and LUGANO. These trials were designed in close alignment with the FDA and informed by the phase 2 DAVIO2 trial, which was the largest trial of an intravitreal TKI to date. LUCIA and LUGANO will evaluate the noninferiority of EYP-1901 administered every 6 months vs aflibercept 2 mg every 8 weeks.
We are working closely with the retina community and recruitment is exceeding expectations, reflecting the excitement for the intravitreal insert. Top-line results are expected in 2026.
Dr. Modi: Can you highlight critical differences between the phase 2 and phase 3 programs in DME and the rationale for making these changes?
Dr. Duker: A protocol for phase 3 trials in DME are currently being discussed. Results and learnings from our positive phase 2 clinical trial program are being leveraged to inform the design. Like the phase 3 trial in nAMD, we expect that a phase 3 DME trial will include a redosing schedule for EYP-1901.
Dr. Modi: Is there anything you want the readers to know about EYP-1901?
Dr. Duker: The intravitreal insert provides a controlled release, with the drug reaching target tissues within hours after injection. Vorolanib is released with zero-order kinetics, which allows for a continuous stable dose of drug over a period of at least 6 months, which could potentially reduce treatment burden for patients with these exudative retinal diseases. The drug is also fully eluted before matrix bioerosion so there are no-free floating drug particles.
We also have the most robust clinical trial program for an intravitreal TKI. To this point, the vorolanib intravitreal insert has been evaluated in more than 190 patients across several indications and has shown a favorable safety and tolerability profile, with no ocular or systemic serious adverse events.
Dr. Modi: Dr. Kaden, the TKI platform can be administered intravitreally and even via the suprachoroidal space. There are 3 programs that have initiated phase 3 studies. If EYP-1901 were to be approved, how do you see yourself clinically using this medication?
Dr. Kaden: There are a few things I think about when considering the use of new drugs. These include the obvious ones: efficacy, durability, and safety. At the moment, we have some excellent treatment options that I would argue have excellent efficacy, safety, and even durability. But I think what we’re potentially looking at with these new TKI therapies is a level of durability that would have seemed impossible just a few years ago. So that’s quite exciting. In terms of safety, our tolerance for treatment complications is low. We have been privileged to have safe therapies as clinical options, so the bar is quite high for new therapies. But there are also other considerations: cost and availability, storage requirements, and ease of administration. Personally, I think we should not underestimate the advantage of an intravitreal injection as compared to other platforms. As a field, we have become incredibly comfortable with this route of administration and perhaps even more importantly, so have our patients. I think it will be a lot easier to imagine transitioning a patient from one intravitreal therapy to another. But I’m excited that we’re always pushing the boundaries of what is possible, both in terms of mechanisms of action and administration techniques.
Dr. Modi: Assuming FDA approval, how will you follow patients in your practice to ensure clinical improvement or worsening? Does this treatment just reduce injection frequency, or does it have the potential to also reduce visit burden?
Dr. Kaden: I imagine that initially, there will ideally be a reduction in treatment frequency, but perhaps not as significant a reduction in visit burden. This is because we will likely want to have interim check-ins to assess how they are doing, ensure there are no unexpected complications, and evaluate drug efficacy. Additionally, we know from the clinical trials that some patients will need breakthrough or rescue treatment, and we don’t want to miss that moment.
However, I am optimistic that as I develop my own experience with the medications (in this hypothetical, FDA-approved future!) and as real-world data from other physicians are released, we’ll become more comfortable with these treatments, which one hopes will lead to fewer in-office visits. In this future, I can also envision the use of home OCT monitoring as an adjunct assistant in managing these patients, which could further reduce the need for interim visits. RP












