Data from a large real-world study, presented at the 2025 American Society of Retina Specialists (ASRS) meeting in Long Beach, California, suggest that newer intravitreal therapies administered in higher volumes do not increase the risk of intraocular pressure (IOP) elevation or glaucoma development in treatment-naïve patients.
Deepak Sambhara, MD, FASRS, a partner and medical director of research at the Eye Clinic of Wisconsin in Wausau, Wisconsin, led the study, which analyzed electronic health records from the Vestrum Health database. The study focused on 3 high-volume therapies that received FDA approval in 2023: aflibercept 8 mg (Eylea HD; Regeneron) for neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME), as well as pegcetacoplan (Syfovre; Apellis Pharmaceuticals) and avacincaptad pegol (Izervay; Astellas Pharma) for geographic atrophy (GA).
“All 3 of those medicines have higher volumes of administration, ranging from 70 µL in the case of aflibercept 8 mg to 100 µL for pegcetacoplan (PEG) or avacincaptad pegol (ACP),” said Dr. Sambhara. “Because these medicines have higher volumes than normal, it’s natural to question whether there is any increased risk of pressure elevation or the development of glaucoma in patients receiving these treatments.”
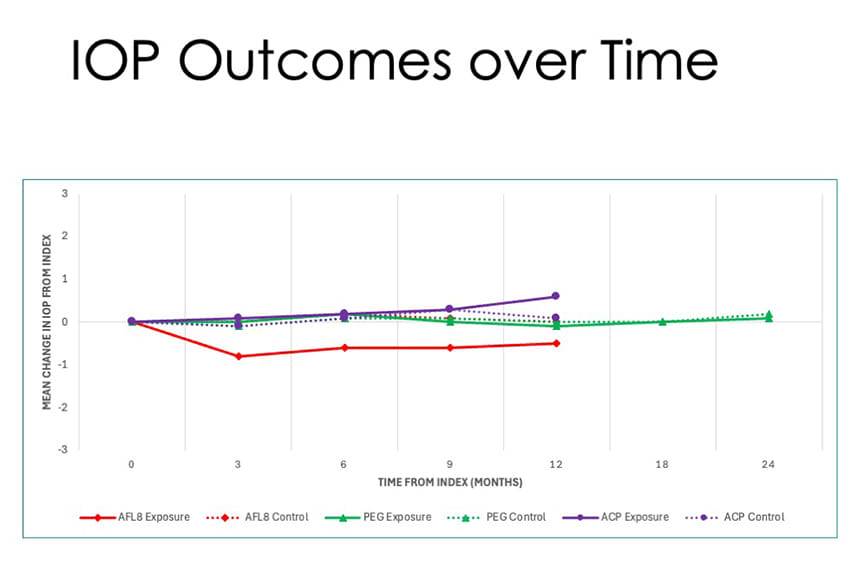
Figure 1. Intraocular pressure remained stable over time, with no significant elevations observed in any treatment group or their respective controls. Mean IOP stayed within 1 mmHg of baseline across all index drugs through 12 months, and through 18 months for the PEG group.
Although phase 3 clinical trials didn't demonstrate an appreciably greater risk of IOP elevation or the development of glaucoma in these patients, Dr. Sambhara emphasized the importance of examining real-world outcomes in a larger sample of patients. “That was the motivation behind the study,” he said.
Study Organization
Using Vestrum Health’s national panel of deidentified electronic health records, which contains more than 1 million eyes, the research team applied strict inclusion and exclusion criteria, censoring eyes with prior ocular trauma, steroid use, therapy switches or multiple concurrent therapies, or a greater than 6-month gap in treatment. Eyes with preexisting glaucoma were also excluded. The remaining eyes (n=3,741) were divided into 6 cohorts: 3 index cohorts receiving aflibercept 8 mg, PEG, or ACP, and 3 control groups treated with standard 50 µL anti-VEGF agents. The control groups were matched by diagnosis, baseline IOP, and injection frequency over a follow-up period.
The researchers followed patients in the matched cohorts over time, looking at both demographics and treatment patterns. Baseline IOP was less than 14 mmHg across all groups, and these were patients with no previous history of glaucoma. “What you can see with demographics is that the baseline IOP across all groups and their control arms were similar, their baseline vision was similar,” said Dr. Sambhara.
Treatment patterns showed that aflibercept 8 mg—the only anti-VEGF agent in the index group—was often used with a treat-and-extend regimen, resulting in fewer injections over time. PEG and ACP were administered at fixed monthly or bimonthly intervals. The different approaches and treatment intervals were reflected in both index and control group data.

Figure 2. Survival plots show no increased risk of developing glaucoma when compared to control groups over time, though these numbers are not statistically significant, noted Dr. Sambhara.
Key Findings
Over the 24-month observation period, Dr. Sambhara reported, IOP remained stable across all 3 treatment groups, regardless of whether patients received aflibercept 8 mg, PEG, or ACP (Figure 1). Mean IOP values were consistent, with differences within 1 mmHg compared to their matched control groups. These differences were not statistically significant, suggesting that the use of higher-volume intravitreal injections does not result in meaningful IOP elevation over time.
Cox proportional hazards analysis further supported these findings. In the aflibercept 8 mg and ACP groups, there was no statistically significant increase in the risk of IOP elevation greater than 5 mmHg; PEG showed a statistically significant risk compared to the control arm (P=.038). “This was an isolated finding, so it’s important not to draw weaving conclusions based on that one data point alone,” said Dr. Sambhara. There was no statistically significant risk of IOP rising above 25 mmHg in any treatment group, and the incidence of newly diagnosed glaucoma did not differ significantly between patients receiving higher-volume agents and their control counterparts (Figure 2). “Thankfully, we again see that there isn't any statistically significant risk of developing glaucoma in any of these groups compared to their comparator arms,” said Dr. Sambhara. “It’s reassuring to know that our data set doesn't indicate that there's an increased propensity to IOP events or the development of glaucoma, which is good.
“At the end of the day, these medicines still need to be studied over time to just make sure of [these findings]. But the real-world data that exist corroborate what we’ve seen in the clinical trials, which is that there is not a higher risk, at least at this point, in developing glaucoma,” he continued (Figure 3). “As newer agents come to the market that have higher than normal volumes that are injected, we need to have longitudinal follow up to better contextualize data and monitor these patients over time.”
Although Dr. Sambhara disclosed consulting relationships with Apellis, Astellas, and Regeneron, he emphasized that this analysis is an independent, pharmaceutical industry–agnostic project, conceived and executed in collaboration with Vestrum Health. RP

Figure 3. Real-world data suggest that the use of higher-volume injectables do not increase a patient’s risk of developing ocular hypertension or glaucoma.








