In May 2024, the US Food and Drug Administration granted de novo marketing authorization for Notal Vision’s Scanly home optical coherence tomography (OCT) imaging device, which enables physicians to monitor progression of neovascular (“wet”) age-related macular degeneration (nAMD) between office visits. Currently, the DRCR Retina Network is conducting Protocol AO (NCT05904028), a study that will compare nAMD treatment guided by home OCT with a more traditional treat-and-extend (T&E) approach (Figure 1, 2, 3, 4). The primary objective of Protocol AO is to determine whether home OCT guidance results in better visual acuity outcomes and/or a lower number of injections over 104 weeks, compared to T&E.
Christina Y. Weng, MD, MBA, a professor of ophthalmology and director of the vitreoretinal diseases and surgery fellowship program at the Baylor College of Medicine in Houston, is the national protocol chair of Protocol AO. During our conversation, which has been edited for length and clarity, Dr. Weng outlined some ways that home OCT could allow a customized treatment regimen for nAMD patients and discussed the real-world challenges of incorporating home OCT into a busy retina practice.
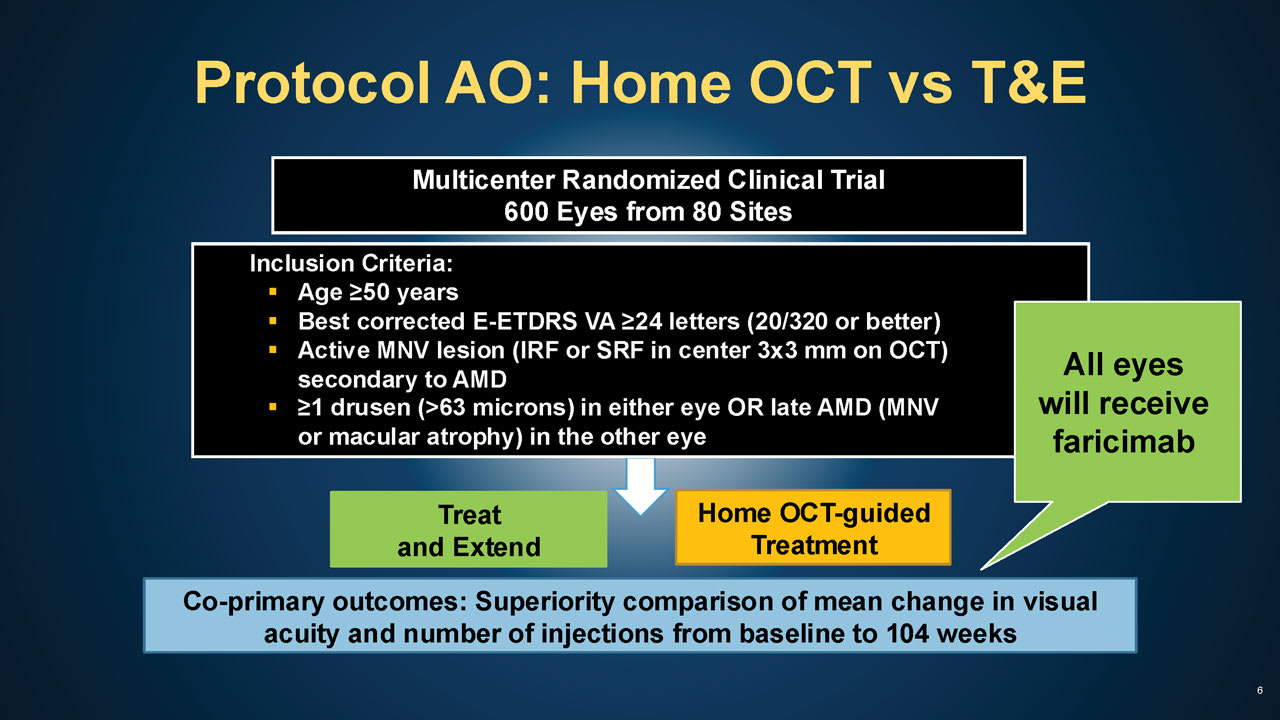
Figure 1. Protocol AO study design. All images courtesy DRCR Retina Network.
Key: E-ETDRS = electronic early treatment diabetic retinopathy study letters; VA = visual acuity; MNV = Macular neovascular membranes; IRF = intraretinal fluid; SRF = subretinal fluid.
Retinal Physician (RP): Can you briefly discuss how the home OCT-guided treatment strategy would work? What advantages does it offer compared to treat-and-extend?
Dr. Weng: Treat-and-extend emerged as a way to find a happy medium between monthly fixed and pro re nata (PRN) dosing. With monthly injections, we achieve great outcomes, but the treatment burden is unsustainable for most; with PRN schedules, patients visit less frequently, but unfortunately may have subpar outcomes in real-world settings. T&E is intended to minimize the number of injections without compromising visual acuity outcomes, and is the most common approach used in nAMD management — but it’s not without shortcomings as it frequently leads to either overtreatment or undertreatment.
Home OCT will bring us one step closer to truly personalized care. I foresee home monitoring technologies changing the patient journey in 2 potential ways. First, it may allow some patients to reduce their treatment burden. Right now, many retina specialists load with monthly injections and then extend or contract treatment intervals by 2-week or 4-week increments. But that means that even if the interval extends out without a single recurrence in disease activity, it may take 6 to 12 months to reach q12 to q16 week intervals. Now imagine an alternative scenario where the patient is dry after 1 or even 2 injections and then is subsequently followed with daily home OCT — he/she may be able to go months before needing to return for the next injection. Meanwhile, the retina specialist can feel confident in that deferral, given the capability to monitor the patient’s OCT scans remotely.
Now consider a second scenario — let’s say you have a patient who returns with recurrent fluid 6 weeks following their last injection. This is always a bit disappointing for both the physician and patient, and we are often left wondering how long that fluid has been present. But with home OCT, we might have seen that this patient’s fluid recurred at 5 weeks and 2 days and therefore brought the patient back a little sooner. Improving the precision of treatment timing is paramount, given growing evidence to suggest that repeated fluid fluctuations might negatively impact visual prognosis; it might also mitigate the risks of festering recurrent disease activity, such as lesion growth, submacular hemorrhage, or other detrimental effects of delayed treatment.
Imagine being able to tailor treatment so that a patient only receives an injection when fluid recurs. Home OCT makes this possible and could be transformative in our management of nAMD and may even allow us to achieve better visual outcomes.
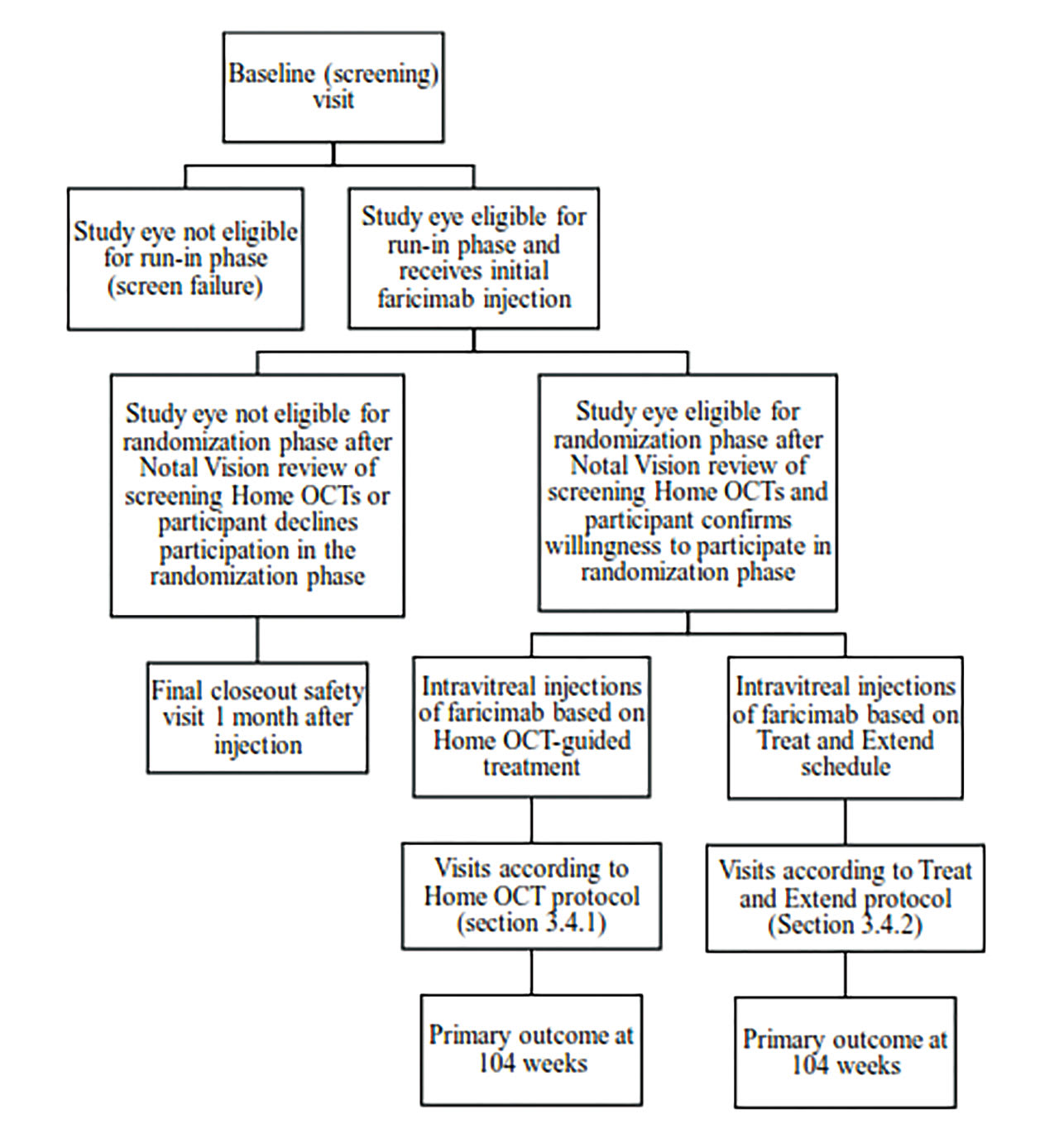
Figure 2. Protocol AO will provide guidance for using home optical coherence tomography (OCT), including fluid thresholds and rules for bringing participants into the office for assessment.
RP: How can daily monitoring with home OCT potentially lead to better outcomes for patients?
Dr. Weng: There are two ways in which home OCT monitoring could render better outcomes: earlier detection of disease activity and facilitation of personalized treatment. Generally speaking, the sooner wet AMD is diagnosed and treated, the better the outcome. Currently we can only identify exudative activity if a patient physically presents for exam and OCT. By monitoring patients on a much more frequent basis, home OCT may allow nAMD to be detected and treated earlier. For patients already diagnosed with nAMD, daily tracking of fluid volumes allows for prompt intervention when a fluid uptick is just starting. This will not only minimize undertreatment, but also minimize harmful fluid fluctuations.
It has been fascinating to see what happens in terms of fluid dynamics following an injection as we’ve never before had this level of insight. Some patients can go long stretches of time without any injections, while others may never get fully dry in between. We’re learning a lot.
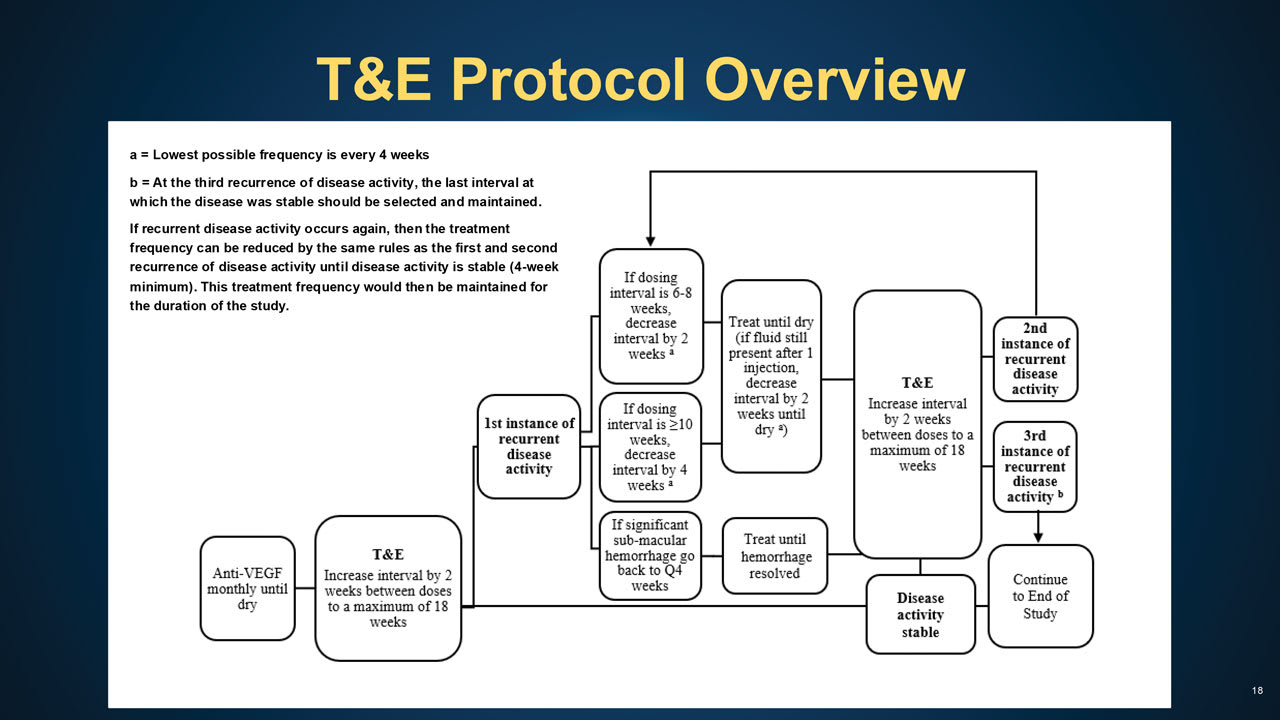
Figure 3. The algorithm for the treat and extend arm of Protocol AO calls for monthly injections until disease activity is stable, then increasing the interval by 2-week increments, to a maximum of 18 weeks, until recurrent disease activity is observed.
RP: One of the primary outcomes for Protocol AO is change in visual acuity from baseline to 104 weeks. How will that be measured?
Dr. Weng: Each time patients come into the office — the frequency of which will depend on their randomization arm — electronic-ETDRS visual acuity testing along with protocol refraction will be performed in the study eye. Low-luminance visual acuity is a secondary outcome that will be measured at baseline and annual visits. Additionally, patients are counseled to contact the office if they experience metamorphopsia or visual changes so that they can return for reevaluation. Thinking ahead, it would be fantastic if home OCT could someday incorporate visual acuity testing capabilities!
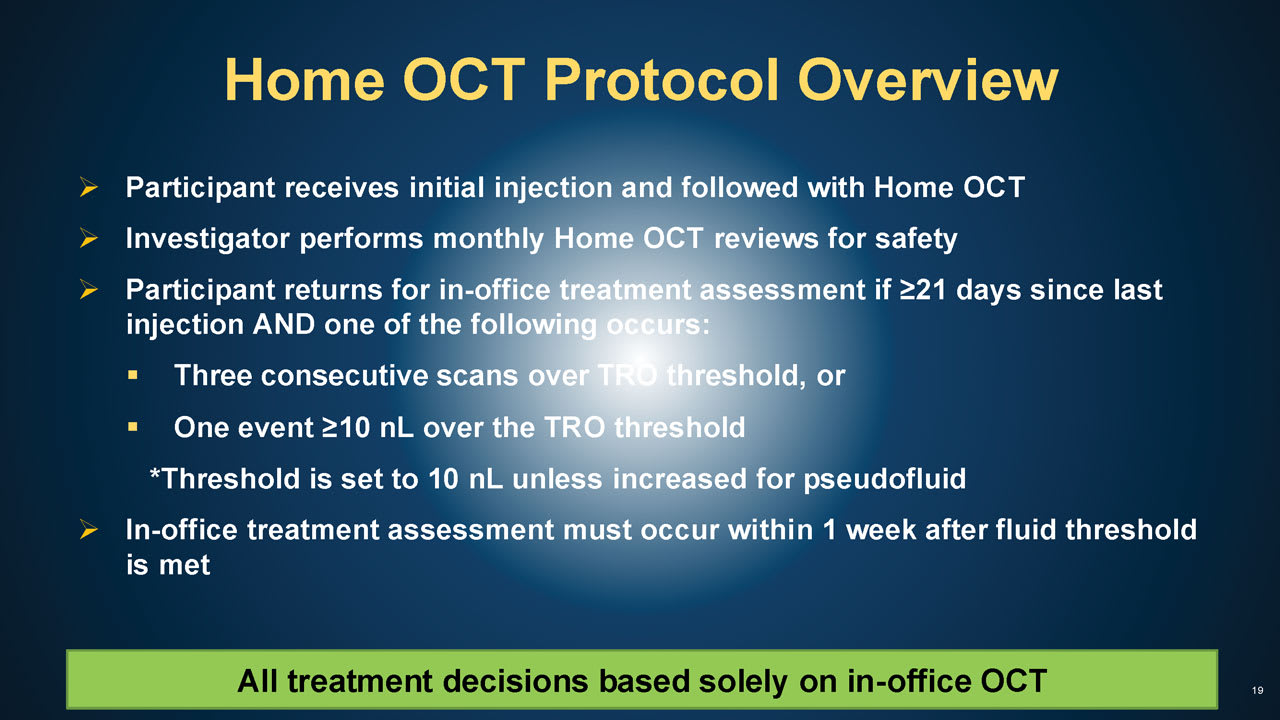
Figure 4. Patients randomized into the home optical coherence tomography (OCT) group will have clinic visits as scheduled or when an increase in hyporeflective spaces on home OCT signals rising fluid. Treatment decisions will be based on in-office OCT.
Key: TRO = total retinal hyporeflective (TRO) spaces.
RP: What challenges do you anticipate in implementing home OCT–guided treatment in clinical practice, especially in terms of patient adherence?
Dr. Weng: Patient adherence is essential since these technologies only work if patients actually use them! Fortunately, Notal Vision has dedicated patient specialists available to assist users on everything from setting up the device to reminding them to scan if they miss a couple of days. This compliance tracking is key because if patients are not regularly scanning, then the potential benefits will not be captured.
If you look across all the small studies that have been done so far with home OCT, compliance has been excellent. For example, in Protocol AK (a pilot study led by Dr. Kevin Blinder and the DRCR Retina Network that informed the design of Protocol AO), patients scanned on average 6.3 times a week, and that number is very similar to what has been published by other groups. Another metric that has been quite consistent across studies is the scan acquisition time of around 45 seconds. So it’s not a heavy time burden, and it has been reassuring to see my own patients in their 70s, 80s, and even 90s successfully operate the device without assistance and obtain high-quality images.
RP: If home OCT–guided treatment proves successful, what steps do you foresee in scaling this approach and incorporating it into standard nAMD management?
Dr. Weng: Home OCT is a disruptive technology so of course workflow integration will need to be addressed. A common concern is how retina specialists will manage patient images and alerts. However, it is important to remember that while patients’ home OCT scans are accessible at any time, the provider does not need to review every single scan; the artificial intelligence-driven platform will do the heavy lifting.
That said, some human capital is still required. For example, when fluid alerts do arise, someone needs to log into the system and review the images. And if a visit is deemed necessary, the clinic staff will need to schedule the patient on short notice. Although these actions are not necessarily time-intensive or labor-intensive, they do require a dedicated workstream. But if the benefits of home OCT are clear, I am confident that we will find a way to make it work.
RP: In your view, what are the potential financial implications for retina clinics?
Dr. Weng: Notal Vision has established 3 Category III home OCT billing codes (0604T, 0605T, and 0606T) for physician and monitoring service provision. The creation of dedicated Category III codes administrated by local Medicare Administrative Contractors (MACs) ensures that the valuation of in-office OCT Category I codes is not impacted. However, these are temporary codes and one may wonder what kind of impact home OCT could eventually have on payments associated with in-office OCT. Time will tell, but because budget neutrality is applied across specialties, introducing a permanent Category I code might not necessarily cannibalize reimbursement rates for similar ophthalmic testing if it is distinct from other imaging/OCT codes.
Another question is whether home OCT will diminish clinic volume and in-office imaging or even treatment-associated revenue. The answer is unknown at this time, but a few points to contemplate: 1) Home OCT may appropriately increase the frequency of visits/injections for a subset of patients currently being undertreated; 2) Home OCT will provide an additional revenue stream because physicians will be able to bill for review of images every 30 days; and 3) Consider that whatever patient slots are vacated due to remote monitoring can be filled with other patients or alternative revenue-generating opportunities. Bottom line, introduction of home OCT might be beneficial from both a patient care and economic standpoint. Advocacy efforts to ensure that retina specialists are compensated fairly for their time and effort associated with this technology will be important in coming years.
RP: What factors influenced the decision to use faricimab in Protocol AO?
Dr. Weng: The Protocol AO development committee carefully considered every available anti-VEGF agent and even considered including multiple agents. Ultimately, we decided that it would be best for all patients to receive the same drug so that an apples-to-apples comparison could be made between treatment approaches. We chose faricimab (Vabysmo; Genentech) because at the time, it was the agent that appeared to offer the potential for greatest durability.
Home OCT will likely be particularly useful when managing patients treated with longer-durability agents. Beyond traditional anti-VEGF injectables, there are gene therapies, tyrosine kinase inhibitors, and other treatments being studied that may allow patients to go 6 months or longer between injections. Most of us would not be comfortable right now letting nAMD patients go for 6 months without an OCT scan to check for disease activity. But if they were scanning daily with home OCT and remained dry/stable, we’d feel a lot more confident about seeing the patient only once or twice a year. This model of care represents a real possibility in the near future given emerging therapeutics! RP









