Radiation therapy of uveal melanoma has been practiced for more than 100 years.1 With high local control rates (95%),2 visually debilitating sequelae have been accepted by the treating oncologist and the patients alike.3 To avoid radiation retinopathy (RR) altogether, alternatives to radiation therapy are being sought. Currently, several promising treatments are being investigated in clinical trials, including a light-activated therapy that can selectively destroy the cell membrane of malignant cells, the use of novel pharmaceuticals to prevent and treat the effects of RR, and an oral drug that blocks a critical step in the molecular pathogenesis of uveal melanoma (Table 1).
CoMpass Trial
Unlike other malignant tumors, choroidal melanoma (CM) is diagnosed clinically, usually without obtaining biopsy. Early detection of indeterminant small lesions (IL) is based on clinical features and/or documented growth.4,5 Therefore, the lesion can be diagnosed either as a high-risk IL or a small CM (both referred to as early-stage disease), depending upon findings and overall expert opinion. This diagnosis uncertainty can delay treatment that is needed to reduce metastatic and mortality risk. Currently, no medication is approved for early-stage primary disease and current therapies, such as plaque brachytherapy, are not tumor-specific and therefore also affect normal ocular tissue, complicating therapy with radiation-associated pathologies.

Figure 1. Delivery and mechanism of action of activation of AU-011 (belzupacap sarotalocan; Aura Biosciences) with a diode laser (689 nm; same as photodynamic therapy), the small molecules selectively destroy the cell membrane of malignant cells. Used with permission from Aura Biosciences.
There is renewed enthusiasm to use light-activated therapy in carefully selected cases of small CM. The CoMpass trial (ClinicalTrials.gov ID: NCT06007690) is a phase 3, randomized, masked, controlled trial to evaluate the efficacy and safety of belzupacap sarotalocan (bel-sar, or AU-011; Aura Biosciences) treatment compared to sham control in subjects with primary IL or small CM.6
Bel-sar consists of synthetic recombinant viral-like particles (VLPs), derived from the human papillomavirus (HPV) conjugated to infrared-activated small molecules. When injected in the suprachoroidal space, the VLP selectively binds to uveal melanoma cells and other solid tumor cells that overexpress heparan sulfate proteoglycans. Upon activation with a diode laser (689 nm; same as photodynamic therapy), the small molecules selectively destroy the cell membrane of malignant cells (Figure 1). Bel-sar acts by catalyzing production of reactive oxygen species, resulting in tumor-specific necrosis with secondary pro-immunogenic response but sparing healthy cells. Previous preclinical and clinical trials have shown safety and efficacy with intravitreal and suprachoroidal bel-sar with tumor control and growth rate reduction.
The trial will enroll at least 85 patients, and possibly up to 105 patients, across 60 centers. Patient included are those with treatment-naïve intraocular IL or small CM (excluding optic nerve proximity or invasion, and extraocular or systemic involvement) with documented growth or de novo presentation at screening. The trial includes 3 arms in a 2:1:2 ratio consisting of cycles of 3 weekly suprachoroidal injections of 80 μg bel-sar with laser, 40 μg bel-sar with laser, or sham with sham laser, respectively, for 3 monthly cycles, followed by observation of 15 months (Figure 2).

Figure 2. Design of the CoMpass trial. Used with permission from Aura Biosciences.
The primary objective is the time to reach the composite endpoint (tumor progression or visual acuity failure), while secondary endpoints include time to tumor progression, tumor thickness growth rate, and visual acuity failure at 15 months. Metastasis and mortality, together with pharmacokinetic parameters, will also be evaluated throughout the study.
Protocol AL
Radiation retinopathy is a frequent complication of ocular radiation therapy, spanning from mild capillary leakage to macular leakage, which may lead to irreversible vision loss. It arises from a chronic inflammatory reaction, leading to the death and dysfunction of retinal capillary endothelial cells. This process starts immediately following radiation therapy but has a delayed clinical presentation. Both short-term intravitreal corticosteroids and intravitreal anti-VEGF therapy have been shown to reduce, albeit partly, these consequences. Currently, the natural history and progression of RR are yet to be fully elucidated, with no proven effective treatment to stop onset or progression of RR.
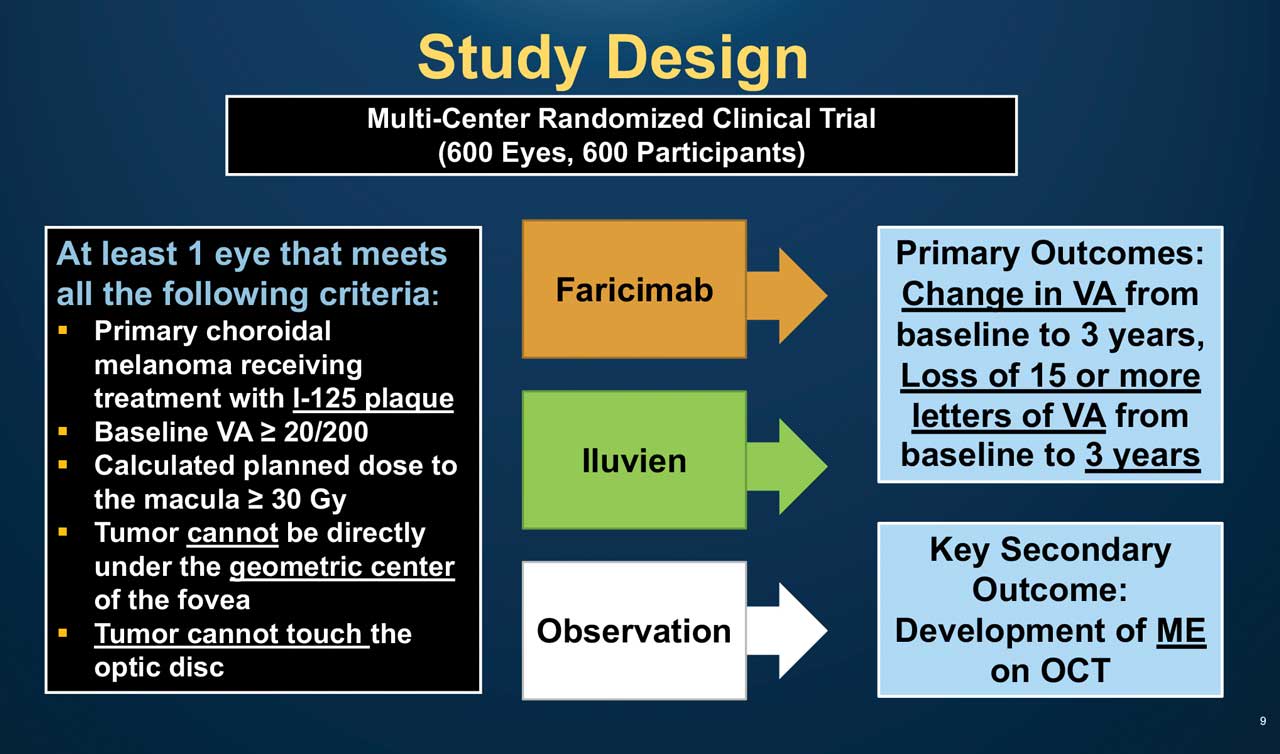
Figure 3. Design of the DRCR Network’s Protocol AL trial. Used with permission from the JAEB Center for Health Research.
A trial is under way to prevent and treat RR using novel pharmaceuticals that are efficacious for management of diabetic retinopathy because of similarities between the 2 conditions. Protocol AL (ClinicalTrials.gov ID: NCT05844982) is a phase 3 randomized clinical trial being conducted by the DRCR Network. It is designed to compare between intravitreal faricimab 6.0 mg (Vabysmo; Genentech), which has a dual anti-Ang2/VEGF mechanism, or fluocinolone acetonide intravitreal implant 0.19 mg (Iluvien; Alimera Sciences), a long-acting corticosteroid, vs observation, for the prevention of visual acuity loss due to RR, following iodine-125 plaque brachytherapy for CM, aiming that treatment with either at the time of radiation exposure, could decrease RR risk and vision loss (Figure 3).7 The trial will include patients with primary uveal melanoma (except iris melanoma) with >20/200 vision, receiving primary therapy with plaque brachytherapy with macular radiation exposure. Patients with primary open-angle or steroid-induced glaucoma, ocular hypertention >25 mmHg, moderate nonproliferative diabetic retinopathy, history of previous vitrectomy, or with other causes that might affect macular edema or visual acuity, will be excluded.
Eligible patients will be randomized, at a 1:1:1 ratio, to either Vabysmo at randomization and every 3 months, Iluvien at randomization and at 24 months, or observation, and will have follow-up visits every 3 months for 3 years. Macular edema will be evaluated from the sixth month after randomization; if macular edema develops, it will be additionally treated with either intravitreal faricimab, Iluvien implant, or both, depending upon initial randomization. Primary outcomes include change in visual acuity from baseline. Key secondary outcomes include assessment of macular edema development, other radiation complication, relevant changes per OCT and OCTA, and safety.
Protocol IDE-196-009
Radiation therapy as a globe-sparing treatment for uveal melanoma is limited by tumor size and location and is intended for tumors up to 10 mm in thickness and/or 16 mm basal diameter, with enucleation usually recommended beyond these limits. Treatments to reduce the size of uveal melanoma, enabling conversion from enucleation to plaque radiotherapy and thus preserving the globe in large tumors, or enabling less radiation exposure during plaque brachytherapy in medium tumors, are needed but are currently not available. Also, current treatment to lower the burden of metastatic disease exist but are at best only palliative.

Figure 4. Darovasertib is an investigational selective inhibitor of protein kinase C, which blocks mutations in GNAQ/GNA11, a genetic driver of uveal melanoma. Used with permission from Ideaya Biosciences.
Darovasertib (IDE96; Ideaya Biosciences) is a potent, selective inhibitor of the protein kinase C (PKC) family that blocks activated PKC signaling in the mutated GNA11 or GNAQ intracellular pathway of cellular survival and proliferation, harbored by a large portion of patients (Figure 4). In previous and ongoing phase 1 trials, darovasertib showed antitumoral effect in metastatic and localized disease (and even tumor shrinkage) with a tolerable safety profile.

Figure 5. Design of the phase 2 study of darovasertib as neoadjuvant or adjuvant therapy in primary uveal melanoma. Used with permission from Ideaya Biosciences.
The IDE-196-009 protocol (ClinicalTrials.gov ID NCT05907954) is a phase 2 multicenter, open-label study of IDE196 in patients with primary uveal melanoma, with no extraocular or systemic disease.8 It includes 2 cohorts, the first with 41 patients offered primary enucleation and the second with 41 patients offered primary plaque brachytherapy, based on tumor characteristics. Each cohort will receive neoadjuvant treatment of 300 mg darovasertib BID (based on previous trials) for a 28-day cycle until the maximal benefit (no change in tumor over 2 cycles) up to 6 cycles, followed by the primary treatment. If neoadjuvant benefit is observed, the patients will be offered 6 cycles of adjuvant treatment (Figure 5). Primary objectives are to evaluate the tolerability and safety of darovasertib, and to evaluate efficacy by the rates of patients converted from enucleation to plaque brachytherapy for the first cohort and comparison of modeled foveal radiation exposure before and after neoadjuvant therapy for the second cohort. Secondary objectives include assessing visual acuity loss, local disease recurrence, metastatic spread, and overall survival. RP

References
1. Echegaray JJ, Bechrakis NE, Singh N, Bellerive C, Singh AD. Iodine-125 brachytherapy for uveal melanoma: a systematic review of radiation dose. Ocul Oncol Pathol. 2017;3(3):193-198. doi:10.1159/000455872
2. Bellerive C, Aziz HA, Bena J, et al. Local failure after episcleral brachytherapy for posterior uveal melanoma: patterns, risk factors, and management. Am J Ophthalmol. 2017;177:9-16. doi:10.1016/j.ajo.2017.01.024
3. Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostication tool. JAMA Ophthalmol. 2016;134(6):615-620. doi:10.1001/jamaophthalmol.2016.0104
4. Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014;121(6):1281-1288. doi:10.1016/j.ophtha.2013.12.014
5. Zabor EC, Raval V, Luo S, Pelayes DE, Singh AD. A prediction model to discriminate small choroidal melanoma from choroidal nevus. Ocul Oncol Pathol. 2022;8(1):71-78. doi:10.1159/000521541
6. Aura Biosciences. Pipeline programs: choroidal melanoma. Accessed August 1, 2024. https://aurabiosciences.com/pipeline-programs/choroidal-melanoma/
7. JAEB Center for Health Research. Protocol AL. Accessed August 1, 2024. https://public.jaeb.org/drcrnet/stdy/598
8. (Neo)Adjuvant IDE196 (darovasertib) in patients with localized ocular melanoma. ClinicalTrials.gov ID NCT05907954. Updated July 11, 2024. Accessed August 2, 2024. https://clinicaltrials.gov/study/NCT05907954










