Digital technologies and artificial intelligence (AI)–based systems are revolutionizing every aspect of our lives. This has been enabled by 2 key developments: the ability to collect data in unique ways using innovative digital sensing technologies and processing these data using AI-powered algorithms. Although other fields of medicine have long used remote monitoring technologies for disease state management, this area is a developing field in retina and ophthalmology. For example, cardiologists have routinely used asynchronous platforms for Holter and pacemaker monitoring for decades. Some platforms have indeed been used in retina for some time, but their uptake has not been ubiquitous. However, changing patient expectations and medical economics, coupled with the development of numerous, increasingly sophisticated AI-based products that are still patient-friendly, portends a paradigm shift in this area.
All forms of telemedicine are currently available to retina specialists. These include synchronous (live), store-and-forward, remote patient monitoring (both for home and in-clinic settings), and wearable or mobile health products. Similarly, multiple disease states can currently be assessed, including nonexudative AMD, neovascular AMD (nAMD), diabetic retinopathy (DR), retinopathy of prematurity (ROP), and other maculopathies. There are several solutions in different phases of development that are being marketed. Three key factors should be considered before adopting these technologies: (1) the unmet need to be addressed, (2) the clinical evidence behind the solution, and (3) ease of integration within current practice workflows. This article will explore a few examples of digital and AI technologies in the retina space and how they stack up against these criteria.
Existing Platforms
Highly effective treatments for nAMD are now available that did not exist 2 decades ago. However, real-world data from the IRIS Registry show only 34% of patients with nAMD have functional vision.1 The registry also shows that long-term outcomes in the real world highly correlate with the visual acuity at the time of detection of nAMD conversion. Because most intermediate AMD patients are seen at an interval of 6 months or more, detection and treatment are often delayed. Delayed detection of conversion to wet AMD is hence a proven unmet need that can be alleviated with more frequent data collection.
ForeseeHome
The ForeseeHome AMD Monitoring Program (Notal Vision) allows patients to monitor changes to their vision using preferential hyperacuity perimetry (PHP) to map the central 14° of visual field. This home-based test detects changes in retinal metamorphopsia using an AI algorithm. Such a change can be an indicator of macular neovascularization and hence conversion from intermediate to wet AMD.
Evidence Behind the Efficacy of ForeseeHome
ForeseeHome is the first and only home monitoring tool cleared by FDA under its 510(k) program and covered by Medicare via dedicated Current Procedural Terminology codes. ForeseeHome’s efficacy was evaluated in the HOME randomized, controlled trial2 as part of the AREDS2 clinical trial. The 1,520-patient study showed ForeseeHome, along with standard care, was significantly better at catching conversions from intermediate to wet AMD compared to standard care alone. The effect was so pronounced that the study reached its statistical significance within 1 year and was stopped early by the Data and Safety Monitoring Committee (DSMC) for high efficacy.
The efficacy of ForeseeHome in the real world has been evaluated using 2 large retrospective studies. A real-world IRIS analysis3 showed that patients using ForeseeHome were able to maintain their functional vision at conversion more than twice as frequently as those on standard of care alone. The ALOFT study4 that involved 5 retina clinics and more than 2,000 patients showed similar real-world results. In addition, the ALOFT study demonstrated that patients continued to maintain the gains of early detection at conversion after years of anti-VEGF therapy.
Clinical Integration of ForeseeHome
A central part of the ForeseeHome AMD Monitoring Program is a dedicated monitoring center that works as a digital health-care provider. Patients are referred to the monitoring center by their physicians. The monitoring center handles patient benefits verifications, device provisioning, training, compliant testing, and technical support. When an AI-based alert (Figure 1) for potential conversion from intermediate to nAMD is generated, the monitoring center informs the clinic so the treating clinician can determine the best course of action, which typically includes an in-office follow-up exam for diagnosis. Not all alerts result in nAMD conversion, but a subanalysis of the ALOFT study showed that “false positives” are a strong indicator of future conversion and can help with more informed, vigilant care for these patients.5 This model of remote patient monitoring fits seamlessly with current practice processes and avoids significant burden on retina clinics. Thus far, nearly 40,000 patients have used ForeseeHome monitoring under this model.

myVisionTrack
The myVisionTrack (mVT) mobile app is an FDA registered Class 1 program that is intended for the detection and characterization of metamorphopsia due to disease or treatment deterioration in patients with maculopathies by tracking the central 3° of visual field. It can be deployed via smartphone, tablet, laptop, or desktop computer. mVT uses hyperacuity testing via an app and portal setup whereby a series of evolving shapes tests patients’ ability to make spatial distinctions. Alerts resulting from crossing thresholds based on preset parameters are sent to providers via email.
In addition to its potential usefulness in detecting conversion from nonexudative to exudative AMD, mVT has also been tested in a Moorfields Eye Hospital cohort study evaluating its functionality in 417 patients already receiving intravitreal injections. mVT was used in this group for disease tracking and to detect fluid recurrence, and 64.3% of patients fulfilled the definition of compliance with utilization of the program.6 These promising results are yet to be validated in a randomized controlled trial.
Macustat
Macustat (RemoniHealth) is an FDA-registered monitoring test of retinal function for patients with chronic retinal conditions, which can be prescribed by the physician. It consists of a trimodal central retinal function scan encompassing (1) the Accustat visual acuity subtest, (2) an electronic Amsler grid test with dynamic segmental interrogation (for the central 5° of foveal function and 10° of parafoveal function) and photoreceptor stress testing, and (3) hyperacuity linear retinal function scanning with 2-dimensional mapping of retinal defects (Figure 2). In cross-sectional studies performed with 25 and 33 patients, the results show strong correlations with in-office visual acuity examinations.7,8
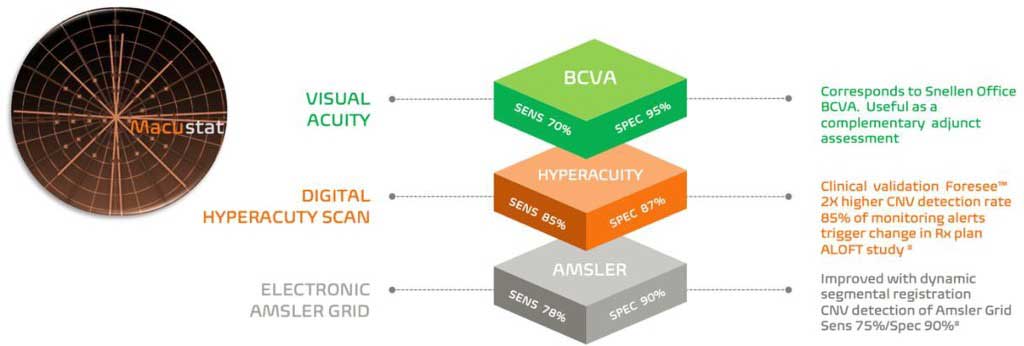
Macustat is a device-agnostic technology and can be accessed on any internet-connected device with a browser and does not require the downloading of any apps. The RemoniHealth Remote Monitoring Platform can serve as an extension of the eye-care practice, whereby remote ophthalmic aides monitor test results and alert provision to the physician. This level of remote monitoring constitutes a fully reimbursable service model via CMS remote physiologic monitoring and principal care management coding.
Heru
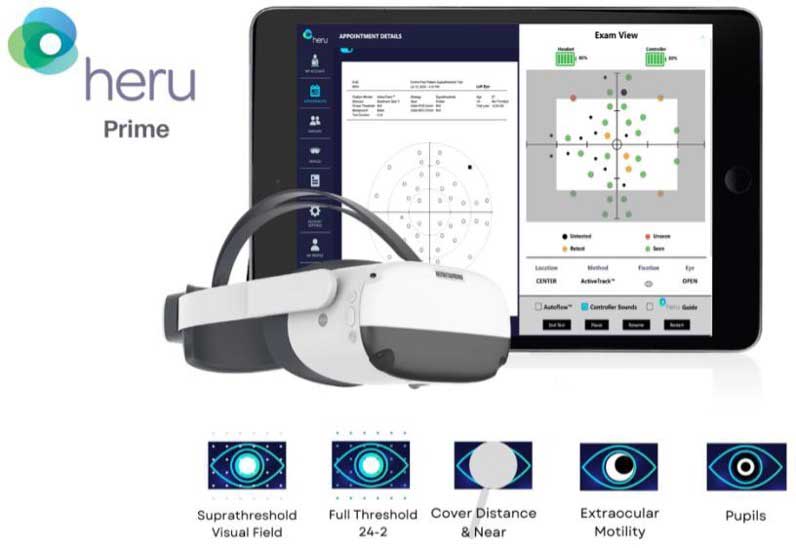
Remote monitoring technologies are not restricted to home monitoring devices. Heru Prime represents a wearable vision diagnostic and augmentation platform with multifunctional applications that can be used as part of the work-up and diagnostic process in the retina clinic. Its functions include full-threshold, suprathreshold, and fast-pattern visual field testing; color vision, contrast sensitivity, and dark adaptation testing; extraocular motility assessment; cover testing; and pupillometry (Figure 3). Use of the wearable device can reduce clinic wait time and exam duration by 32% to 33% based on time and motion studies.9
Retinopathy of Prematurity Telemonitoring
In 2018, the American Academy of Ophthalmology and the American Academy of Pediatrics released a joint policy statement defining the standards for screening of infants with ROP. They endorsed the use of wide-angle fundus photography as a preliminary screening tool that could be used in combination with binocular indirect ophthalmoscopy (BIO).10 This represented the culmination of many studies which have demonstrated the success of telemonitoring to identify referral/treatment warranted disease.11-13 Some examples of telescreening platforms include NeoLight’s ICON camera coupled with the Connect viewing software, the RetCam Envision digital imaging system using the DataLink software, and the integrated TeleROP camera and software system.
The most common application of telescreening for ROP employs an asynchronous, or store-and-forward, approach. This technique uses a trained individual, often a nonphysician, who obtains weekly or biweekly fundus photographs using a wide-angle contact fundus camera. These images are uploaded to a secure server and an expert grader evaluates the fundus images within 24 hours. When the disease is worsening, image quality precludes adequate grading, or the infant completes screening criteria, a bedside examination with BIO is triggered. Using this approach, the identification of referral/treatment warranted disease approaches 100% sensitivity and specificity and rivals that of the BIO.
Concrete advantages of asynchronous ROP screening include the ability to evaluate infants over large geographic distances and to potentially mitigate concerns regarding the growing need for ROP screeners. However, other advantages of the store-and-forward approach may become more apparent as AI platforms become incorporated into ROP screening. These platforms offer the potential to facilitate ROP screening by removing the subjective nature of ROP diagnostics. This path forward will likely include the development and implementation of feature-extraction based tools and machine learning algorithms capable of predicting high-risk cases prior to the development of significant disease. If successful, ROP screening may look very different in the years to come.
Remote Monitoring in Diabetic Retinopathy
Diabetic retinopathy is a common complication of diabetes that demands consistent monitoring to prevent vision loss. Remote monitoring, incorporating innovative tools such as AI-based staging of DR, has emerged as a transformative approach. DR is a progressive condition that can lead to irreversible vision impairment if not detected and managed in its early stages. Regular monitoring is crucial to identify changes promptly, allowing for timely interventions to prevent or slow progression of the disease.
The integration of AI in DR monitoring has introduced advanced algorithms capable of staging the disease based on retinal imaging. These AI tools analyze retinal photographs to identify signs of DR, enabling a more efficient and precise assessment of disease severity.
Patients undergo retinal imaging, typically through fundus photography, which captures detailed images of the retina, often in primary care settings. AI algorithms then analyze these images, categorizing the severity of DR. The results are shared with healthcare professionals, facilitating remote monitoring, and allowing for timely adjustments to the patient’s treatment plan.
The outcomes from the use of AI-based staging in DR have shown promising results.14,15 The accuracy and speed of AI algorithms contribute to early detection and classification of the disease, enhancing the efficiency of remote monitoring programs. Timely identification of DR stages allows for proactive management, potentially reducing the risk of vision loss in diabetic patients.
Currently there are 2 FDA-cleared fully autonomous AI systems to screen for different stages of DR: (1) LumineticsCore (Digital Diagnostics, Inc.) indicated for use with Topcon NW400, and (2) EyeArt (Eyenuk, Inc.) indicated for use with Topcon NW400, Canon CR-2 AF, and Canon CR-2 AF plus.
Future State Technologies
Home Optical Coherence Tomography for Management of nAMD
The treatment of nAMD requires continuous management of patients with anti-VEGF intravitreal injections given at a dosing regimen ranging from 4 to 12 weeks in most cases. The heterogeneity in disease makes it difficult to optimize exact dosing regimens. This puts a heavy burden both on the practices along with patients and their caregivers. There is a clear unmet need to personalize nAMD treatment by better understanding disease dynamics in between office visits.
Home optical coherence tomography (OCT) is a promising technology that allows patients to acquire OCT images at home that are then processed using AI. The physician can then decide to invite a patient for in-office evaluation and treatment based on this home-acquired data.
Evidence Behind Efficacy of Home Optical Coherence Tomography
The ability for patients to self-acquire OCT images in an adherent fashion has been shown in DRCR Retina Network’s protocol AK16 along with other cross-sectional and longitudinal studies.17,18 The study also showed a strong correlation between the AI-driven fluid quantification and the expert graders. A subsequent study involving 15 patients provided the original evidence that home OCT can be used to extend retreatment intervals for patients with nAMD.19 Home OCT as of this writing has not yet received clearance from regulatory bodies for regular clinical use. DRCR Retina Network’s protocol AO is underway and is the first randomized controlled trial to compare home OCT with treat-and-extend based management.
Home OCT management of patients is based on the same model as ForeseeHome, wherein a dedicated monitoring center is responsible for key elements of patient monitoring. This model has again shown a high adherence rate in terms of at-home OCT scans performed by patients.
Conclusion
The landscape of remote monitoring in retinal care is evolving rapidly. New platforms continue to arise and can serve as complements to current retinal clinic structures. These can yield earlier disease detection, improved outcomes, and tailored treatment regimens, while also optimizing clinic efficiency. Physicians should carefully evaluate these platforms to assess which ones are best suited for any given practice. RP
Hear discussion of this article on the Retina Podcast at retinapodcast.com.
References
- Ho AC, Kleinman DM, Lum FC, et al. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: an analysis of the IRIS registry. Ophthalmic Surg Lasers Imaging Retina. 2020;51(11):633-639. doi:10.3928/23258160-20201104-05
- Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design - HOME Study report number 1. Contemp Clin Trials. 2014;37(2):294-300. doi:10.1016/j.cct.2014.02.003
- Ho AC, Heier JS, Holekamp NM, et al. Real-world performance of a self-operated home monitoring system for early detection of neovascular age-related macular degeneration. J Clin Med. 2021;10(7):1355. doi:10.3390/jcm10071355
- Mathai M, Reddy S, Elman MJ, et al. Analysis of the long-term visual outcomes of ForeseeHome remote telemonitoring: the ALOFT study. Ophthalmol Retina. 2022;6(10):922-929. doi:10.1016/j.oret.2022.04.016
- Ho AC, Schechet SA, Mathai M, et al. The predictive value of false-positive ForeseeHome alerts in the ALOFT study [published correction appears in Ophthalmol Retina. 2023 Apr;7(4):371]. Ophthalmol Retina. 2023;7(2):196-198. doi:10.1016/j.oret.2022.10.009
- Korot E, Pontikos N, Drawnel FM, et al. Enablers and barriers to deployment of smartphone-based home vision monitoring in clinical practice settings. JAMA Ophthalmol. 2022;140(2):153-160. doi:10.1001/jamaophthalmol.2021.5269
- Chen E, Mills M, Gallagher T, et al. Remote patient monitoring of central retinal function with MACUSTAT: A multi-modal macular function scan. Digit Health. 2022;8:20552076221132105. doi:10.1177/20552076221132105
- Chen EP, Mills M, Gallagher T, et al. Remote vision testing of central retinal acuity and comparison with clinic-based Snellen acuity testing in patients followed for retinal conditions. Digit Health. 2023;9:20552076231180727. doi:10.1177/20552076231180727
- Internal Heru Time and Motion Evidence Generation Study, 2023.
- Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity [published correction appears in Pediatrics. 2019 Mar;143(3):]. Pediatrics. 2018;142(6):e20183061. doi:10.1542/peds.2018-3061
- Lorenz B, Spasovska K, Elflein H, Schneider N. Wide-field digital imaging based telemedicine for screening for acute retinopathy of prematurity (ROP). Six-year results of a multicentre field study. Graefes Arch Clin Exp Ophthalmol. 2009;247(9):1251-1262. doi:10.1007/s00417-009-1077-7
- Dai S, Chow K, Vincent A. Efficacy of wide-field digital retinal imaging for retinopathy of prematurity screening. Clin Exp Ophthalmol. 2011;39(1):23-29. doi:10.1111/j.1442-9071.2010.02399.x
- Wang SK, Callaway NF, Wallenstein MB, Henderson MT, Leng T, Moshfeghi DM. SUNDROP: six years of screening for retinopathy of prematurity with telemedicine. Can J Ophthalmol. 2015;50(2):101-106. doi:10.1016/j.jcjo.2014.11.005
- Abràmoff MD, Lou Y, Erginay A, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. 2016;57(13):5200-5206. doi:10.1167/iovs.16-19964
- Lim J, Bhaskaranand M, Ramachandra C, Bhat S, Solanki K, Sadda S. Artificial intelligence screening for diabetic retinopathy: analysis from a pivotal multi-center prospective clinical trial. Presented at the 2019 Association for Research in Vision and Ophthalmology Imaging in the Eye Conference; Vancouver, Canada; 2019.
- Blinder KJ, Calhoun C, Maguire MG, et al. Home OCT imaging for newly diagnosed neovascular age-related macular degeneration: a feasibility study. Ophthalmol Retina. 2024;8(4):376-387. doi:10.1016/j.oret.2023.10.012
- Liu Y, Holekamp NM, Heier JS. Prospective, longitudinal study: daily self-imaging with home OCT for neovascular age-related macular degeneration. Ophthalmol Retina. 2022;6(7):575-585. doi:10.1016/j.oret.2022.02.011
- Kim JE, Tomkins-Netzer O, Elman MJ, et al. Evaluation of a self-imaging SD-OCT system designed for remote home monitoring. BMC Ophthalmol. 2022;22(1):261. doi:10.1186/s12886-022-02458-z
- Clark Home OCT guided management of neovascular AMD. Presented at the 2023 Annual Meeting of American Academy of Ophthalmology; San Francisco, CA; 2023.










