The Beckman classification of age-related macular degeneration (AMD), based on color fundus photography (CFP), offers an accessible, easy-to-determine severity scale.1 In the Beckman classification, AMD is categorized as early, intermediate, or late-stage disease and the 2 late forms of AMD are neovascular (nAMD) and geographic atrophy (GA).
The past decade has seen interventional studies aiming to slow the progression of disease, but only once GA was already present. In these trials, atrophy was defined on fundus autofluorescence (FAF) imaging, not CFP, with the primary aim being to slow the rate of growth in the area of such lesions.2,3 Using FAF to define the area of atrophy allowed more accurate delineation of the lesions than CFP, thus enabling more accurate assessment of areas of atrophy and their change over time.
There is still a strong desire to intervene earlier in AMD, before there is irreversible imaging evidence of significant cell death, and with it, concurrent permanent functional loss. However, the time to progress from intermediate AMD to late-stage AMD is variable but can be many years or even decades. As such, an early intervention study aiming to show progression to late AMD, as currently defined, would need to be prohibitively lengthy and/or large to show a clinically meaningful treatment effect, such that it is just not an attractive nor feasible proposition.
This predicament has been a major impediment to advancing interventions that target early stages of AMD and an earlier endpoint that is approved by regulatory bodies would remove this major barrier. This new efficacy outcome of an intervention would be able to be determined through studies involving acceptable participant numbers and conducted over a reasonable number of years.
Defining a Surrogate Endpoint
An early atrophic endpoint that could act as a surrogate endpoint for the late atrophic stage of GA would ideally need to fulfill all the requirements of a surrogate. It has been proposed that a potential surrogate needs to satisfy 4 criteria: (1) a significant effect of treatment on the surrogate; (2) a significant effect of treatment on the clinical endpoint (GA); (3) a significant correlation between the surrogate and clinical endpoint; and (4) the effect of treatment on the clinical endpoint becomes nonsignificant after accounting for the surrogate endpoint, indicating that the effect of treatment on the clinical endpoint can be captured by the surrogate.4
While we await a treatment that could be used to fulfill some of these criteria for a surrogate, it is possible to work toward establishing a significant correlation between an early biomarker that might act as a surrogate endpoint and the clinical endpoint of GA. Further validation of the surrogate endpoint would require the availability of an effective preventative treatment demonstrating efficacy, which currently remains challenging in AMD, given the lack of the surrogate endpoint being a major impediment for the conduct of such preventative treatment trials, thus setting up a vicious cycle.
Identifying a Potential Surrogate Endpoint in Atrophic AMD
Optical coherence tomography (OCT) imaging has enabled observation of the early signs of atrophy that are not clinically visible or detectable on CFP, such as described in incomplete and complete RPE and outer retinal atrophy (iRORA and cRORA).5,6 A decade ago, our group followed a large cohort of individuals with large drusen in at least 1 eye, with or without pigmentary changes, with the aim of identifying those anatomical changes on OCT that preceded the onset of atrophic AMD. The early OCT signs that we found to indicate that the atrophic process had begun were identified and we gave these features the term nascent GA (nGA), where the definition of “nascent” is that which is just coming into existence and beginning to display signs of future potential.7 This term encapsulated the OCT features we observed in this longitudinal natural history cohort.
For nGA to be present, there needs to be 1 or both of the following features: (1) subsidence of the inner nuclear layer (INL) and outer plexiform layer (OPL) and/or (2) a hyporeflective wedge-shaped band within Henle’s nerve fiber layer as indicative of photoreceptor loss (Figure 1).
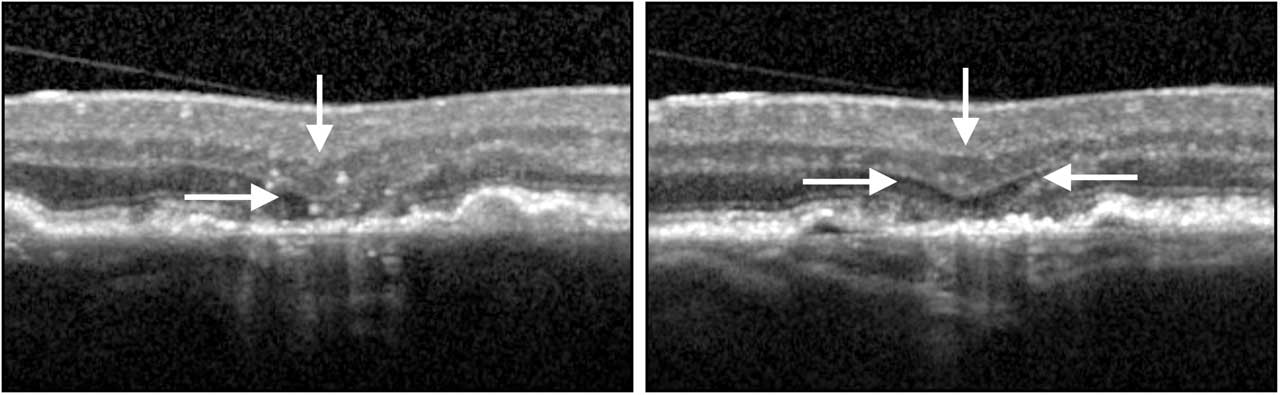
These areas of nGA can be observed on OCT without any indication of their presence on CFP. On FAF imaging, nGA can appear as either as an area of hyperautofluorescence or hypoautofluorescence, but are not seen as well-demarcated areas of hypoautofluorescence.8 As such, in eyes with AMD that is not late stage, it is possible to separate, on the basis of the OCT image, eyes that progressed to show the early signs of atrophy from those that have not.
To determine how well nGA behaved as a potential surrogate endpoint, we subsequently examined a different cohort of individuals with bilateral large drusen and without nGA at baseline to establish the risk of progression to the current Beckman classification of late atrophic AMD (ie, GA). Having followed the cohort with 6-month follow-up intervals for 3 years, we established that eyes with nGA had a 78-fold increased rate of progression to GA as compared to eyes that did not develop nGA.9 Ninety-one percent of the variance in progression to GA was explained by nGA, suggesting that a strong correlation exists between this OCT biomarker and the conventional clinical endpoint of GA.9 In this cohort, the probability of a person with bilateral large drusen developing nGA in either eye over a 36-month period was 24%, which was a substantially higher number of endpoints than those that progressed to GA, which had a 13% probability. A similar increased risk was also reported by an independent group, where having nGA at baseline was associated with a 56-fold increased risk of developing GA over an average follow-up period of 9 years.10
These findings would therefore mean that, in any trial that allowed for a combined atrophic endpoint, using either nGA or GA, there would be more endpoints expected than when using late-stage GA alone, thus enabling smaller, shorter interventions studies aiming to identify efficacy differences between the arms of a study. Indeed, we used nGA and GA as a combined atrophic endpoint in our 3-year randomized nanosecond laser trial, where we achieved many more endpoints with this strategy.11
Other Features of a Robust Endpoint
For any OCT features that are required to be present to determine an endpoint, it is important to determine the ability of reading centers to consistent identify and grade these features as being present or absent. To determine this, we undertook an exercise with 6 international reading centers, where readers were asked to assess early OCT signs that are often seen when atrophy begins, such as those seen in iRORA or cRORA as well as nGA.5,6 We found that the specific features that define nGA — OPL and INL subsidence, and/or the hyporeflective band — had substantial inter-reader agreement, suggesting that it will be possible to reliably identify these features of nGA. Agreement on RPE features has less agreement between graders.12 Several groups are now working toward an automated algorithm that can detect nGA, either in a fully automated or semiautomated fashion, reducing the need for laborious grading of each OCT B-scan.13
When considering the functional correlates of nGA, the macular visual sensitivity, as measured using microperimetry, is on average lower over regions of nGA than over other nonatrophic features in eyes with drusen, but are not yet areas of absolute scotomas that typically characterize areas of GA.14 More targeted, high-density microperimetry testing over regions of nGA enabled the detection of a greater extent of visual sensitivity abnormalities than revealed with standard microperimetry.15
The Way Forward
Although rigorous statistical validation of a surrogate endpoint remains challenging, given the requirement for an effective treatment showing efficacy to establish a true surrogate, a pragmatic approach of considering the biological plausibility of a potential surrogate endpoint is necessary. The accumulating body of evidence highlights how nGA has been observed to precede and be strongly associated with an increased risk of developing GA, but it is characterized by less severe structural and functional changes than those associated with frank GA.
Prevention of photoreceptor loss has been considered a clinically meaningful endpoint by the United States Food and Drug Administration, given the established link between photoreceptor loss and visual function.16 Histologically, regions of subsidence of the OPL and INL and the wedge-shaped hyporeflective wedge in Henle’s nerve fiber layer are seen as areas of photoreceptor loss.5 Nascent GA, or similarly robust features signifying photoreceptor cell death, may well be able to function as a “structural line in the sand,” where stopping them from occurring in a cohort of patients with intermediate AMD will be seen as a sufficiently robust outcome for early interventional trials. If this were the case, surrogates such as nGA could be incorporated into novel trial designs as we begin to see attention turn to interventions that aim to start early in the GA disease process. RP
Editor’s note: Hear discussion of this article on the Retina Podcast at www.retinapodcast.com.
References
1. Ferris FL 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. doi:10.1016/j.ophtha.2012.10.036
2. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402(10411):1434-1448. doi:10.1016/S0140-6736(23)01520-9
3. Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402(10411):1449-1458. doi:10.1016/S0140-6736(23)01583-0
4. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431-440. doi:10.1002/sim.4780080407
5. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3 [published correction appears in Ophthalmology. 2019 Jan;126(1):177]. Ophthalmology. 2018;125(4):537-548. doi:10.1016/j.ophtha.2017.09.028
6. Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: classification of atrophy meeting report 4. Ophthalmology. 2020;127(3):394-409. doi:10.1016/j.ophtha.2019.09.035
7. Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121(12):2415-2422. doi:10.1016/j.ophtha.2014.06.034
8. Wu Z, Luu CD, Ayton LN, et al. Fundus autofluorescence characteristics of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(3):1546-1552. doi:10.1167/iovs.14-16211
9. Wu Z, Luu CD, Hodgson LAB, et al. Prospective longitudinal evaluation of nascent geographic atrophy in age-related macular degeneration. Ophthalmol Retina. 2020;4(6):568-575. doi:10.1016/j.oret.2019.12.011
10. Ferrara D, Silver RE, Louzada RN, Novais EA, Collins GK, Seddon JM. Optical coherence tomography features preceding the onset of advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(9):3519-3529. doi:10.1167/iovs.17-21696
11. Guymer RH, Wu Z, Hodgson LAB, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2019;126(6):829-838. doi:10.1016/j.ophtha.2018.09.015
12. Wu Z, Pfau M, Blodi BA, et al. OCT signs of early atrophy in age-related macular degeneration: interreader agreement: classification of atrophy meetings report 6. Ophthalmol Retina. 2022;6(1):4-14. doi:10.1016/j.oret.2021.03.008
13. Yao H, Wu Z, Gao SS, et al. Deep learning approaches for detecting of nascent geographic atrophy in age-related macular degeneration. Ophthalmol Sci. 2023;4(3). doi https://doi.org/10.1016/j.xops.2023.100428.
14. Wu Z, Ayton LN, Luu CD, Guymer RH. Microperimetry of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;56(1):115-121. doi:10.1167/iovs.14-15614
15. Wu Z, Hodgson LAB, Guymer RH. Targeted high-density microperimetry testing of nascent geographic atrophy in age-related macular degeneration. Ophthalmol Sci. 2023;4(2):100419. doi:10.1016/j.xops.2023.100419
16. Csaky K, Ferris F 3rd, Chew EY, Nair P, Cheetham JK, Duncan JL. Report from the NEI/FDA endpoints workshop on age-related macular degeneration and inherited retinal diseases [published correction appears in Invest Ophthalmol Vis Sci. 2017;58(10):3960]. Invest Ophthalmol Vis Sci. 2017;58(9):3456-3463. doi:10.1167/iovs.17-22339










