Intravitreal injection therapy of anti‒vascular endothelial growth factor (anti-VEGF) continues to be one of the most effective and common procedures performed by vitreoretinal specialists. Anti-VEGF therapies treat a wide spectrum of retinal diseases, including neovascular AMD (nAMD), diabetic retinopathy (DR), diabetic macular edema (DME), retinal vein occlusion (RVO), and myopic choroidal neovascularization (CNV).1 However, these injections come with a high treatment burden, requiring patients to have frequent appointments and potentially arrange for transportation, time off of work, or high out-of-pocket expenses. Additionally, patients can complain of anxiety prior to injections and discomfort after intravitreal injections, which can often take days to improve.2
Gene therapy is an exciting and promising space that could help to alleviate some of this treatment burden. The eye is considered a prime target for gene therapy given its relatively immune-privileged state, ease of delivery, and ability to directly monitor outcomes of therapy with imaging.3 Gene therapy can be delivered via several different routes, including intravitreal, suprachoroidal, and subretinal via vitrectomy surgery.3
The delivery of genetic material inside the target cell is typically achieved using viral or nonviral methods. Viral methods include lentivirus and retroviruses that integrate directly into the host genome, or adenoviruses and herpesviruses that integrate as extrachromosal episomes.3,4 The largest downside of lentiviruses and retroviruses is the ability to cause insertional mutagenesis, which could compromise the target’s cell viability or cause continuous replication and formation of tumors.3,4 Adenoviral vectors are double-stranded DNA viruses that can infect dividing and nondividing cells, but the genetic material remains in episomes and therefore reduces the risk of insertional mutagenesis.3,5 However, this also means that the lack of integration into the main genome may result in dilution of effect over time as the cells continue to divide.3,5 Additionally, the host immune response could clear cells that have been transduced, resulting in elimination of effect, or may cause inflammation in the host.3,5 In ophthalmic gene therapy, adeno-associated viral (AAV) vectors are commonly used. These are nonpathogenic because they require a helper virus for replication. Certain serotypes have a tropism for retinal tissues and are able to transduce cells of the RPE while mostly evading the immune response. These remain episomal similar to adenoviral vectors.3,6
Currently, the only FDA-approved approved ophthalmic gene therapy is voretigene neparvovec-rzyl, also known as Luxturna (Spark Therapeutics). This was approved in 2017 for the treatment of biallelic RPE65 mutation‒associated retinal dystrophy, delivered via subretinal route using an AAV vector.7 The approval of voretigene neparvovec-rzyl represented the first treatment option for patients with RPE65 deficiency and marked a turning point in ocular gene therapy.
There has been increasing focus on gene therapy for common retinal diseases that are treated with intravitreal injections, because these conditions comprise the bulk of patient visits to the retina clinic. There are numerous trials using gene therapy for nAMD, including subretinal gene therapy,8 intravitreal gene therapy,9 and suprachoroidal therapy.10 There are also gene-therapy trials for dry AMD.11 This article will focus on current gene-therapy trials for diabetic retinopathy (DR).
Clinical Trials for Gene Therapy in Diabetic Retinopathy
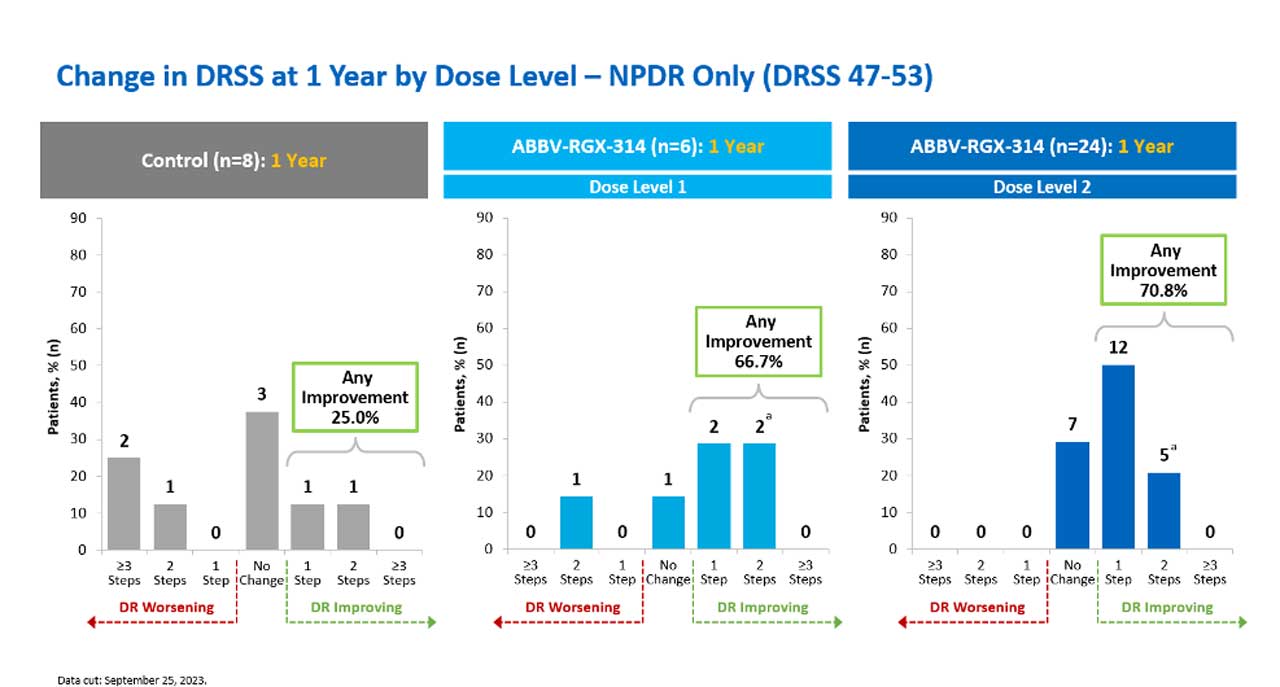
There are currently 2 major trials in the United States using gene therapy for diabetic retinopathy and diabetic macular edema. The first trial is the ALTITUDE study, sponsored by Regenxbio and AbbVie, studying ABBV-RGX-314, which is an AAV8 vector that encodes an antibody designed to inhibit VEGF.12 The phase 2 1-year results of 2 of 3 dosing levels in this trial were recently announced at the 2023 American Academy of Ophthalmology meeting. In ALTITUDE, ABBV-RGX-314 was evaluated in 3 ascending doses for the treatment of DR without center-involved diabetic macular edema (CI-DME) using an in-office suprachoroidal delivery system.12 ABBV-RGX-314 was well tolerated with no drug-related serious adverse events. There was a dose-dependent increase in episcleritis and intraocular inflammation (IOI) events, all of which were graded as mild and resolved with topical corticosteroids. In patients who received dose level 2, 70.8% achieved at least 1 step improvement on the Diabetic Retinopathy Severity Scale (DRSS) as compared to 25.0% in the control group (Figure 1). None of the patients had greater than 2-step DRSS worsening in the treatment group as compared to 37.5% in the control group, and ABBV-RGX-314 reduced vision-threatening events by 89% compared to control.12
The second trial is the SPECTRA clinical trial for DME, which is a phase 2 trial looking at intravitreal 4D-150 in patients with DME.13 4D-150 is produced by 4DMT and is an intravitreal gene therapy using an evolved intravitreal vector, R100, and a transgene cassette that expresses both an aflibercept-lke protein and VEGF-C inhibitor RNAi, inhibiting VEGF A, B, C, and PIGF. In this trial, patients are randomized 1:1:1 to receive a single intravitreal injection of either 4D-150 in 2 different doses, vs an aflibercept control arm.13 The primary endpoint is the annualized number of aflibercept injections in the study eye, and secondary endpoints include incidence and severity of adverse events, changes from baseline in BCVA and CST, and percentage of subjects with a greater than 2 and 3 step DRSS improvement from baseline. The first patients were enrolled in September 2023 and data from SPECTRA is expected during 2024.13
Summary
Gene therapy remains a promising new area that could help alleviate treatment burden in the vitreoretinal disease landscape. While gene therapy has limitations, it could represent a way to deliver meaningful therapies to patients that can last for longer periods of time. Furthermore, gene therapy in ocular diseases represents some of the first gene therapies in medicine and may serve as a platform for the development of gene therapy in other systemic diseases. RP
Editor’s note: Listen to discussion of this article on the Retina Podcast at retinapodcast.com.
References
- Cornel S, Adriana ID, Mihaela TC, et al. Anti-vascular endothelial growth factor indications in ocular disease. Rom J Ophthalmol. 2015;59(4):235-242.
- Yiallouridou C, Acton JH, Banerjee S, Waterman H, Wood A. Pain related to intravitreal injections for age-related macular degeneration: a qualitative study of the perspectives of patients and practitioners. BMJ Open. 2023;13(8):e069625. doi:10.1136/bmjopen-2022-069625
- Moraru AD, Costin D, Iorga RE, Munteanu M, Moraru RL, Branisteanu DC. Current trends in gene therapy for retinal diseases (Review). Exp Ther Med. 2022;23(1):26. doi:10.3892/etm.2021.10948
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12(3):348-353. doi:10.1038/nm1365
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93(24):13565-13570. doi:10.1073/pnas.93.24.13565
- Schön C, Biel M, Michalakis S. Retinal gene delivery by adeno associated virus (AAV) vectors: strategies and applications. Eur J Pharm Biopharm. 2015;95:343-352. doi: 10.1016/j.ejpb.2015.01.009.
- Maguire AM, Bennett J, Aleman EM, Leroy BP, Aleman TS. Clinical perspective: treating RPE65-associated retinal dystrophy. Mol Ther. 2021;29(2):442-463. doi:10.1016/j.ymthe.2020.11.029
- AbbVie. Pivotal 2 study of RGX-314 gene therapy in participants with nAMD (ASCENT). Clinicaltrials.gov identifier: NCT05407636. Updated August 21, 2023. Accessed April 19, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05407636.
- 4D Molecular Therapeutics. 4D-150 in patients with neovascular (wet) age-related macular degeneration. Clinicaltrials.gov identifier: NCT05197270. Updated February 16, 2023. Accessed April 19, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05197270.
- AbbVie. Gene therapy administered in the suprachoroidal space for participants with neovascular age-related macular degeneration (nAMD) (AAVIATE). Clinicaltrials.gov identifier: NCT04514653. Updated May 22, 2023. Accessed April 19, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04514653.
- Janssen Pharmaceuticals. A study to evaluate intravitreal JNJ-81201887 (AAVCAGsCD59) compared to sham procedure for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Clinicaltrials.gov identifier: NCT05811351. Updated March 27, 2023. Accessed April 19, 2024. https://clinicaltrials.gov/study/NCT05811351.
- REGENXBIO presents positive one year data from phase II ALTITUDE Trial of ABBV-RGX-314 for treatment of diabetic retinopathy using suprachoroidal delivery. News release. November 3, 2023. Accessed April 19, 2024. https://www.prnewswire.com/news-releases/regenxbio-presents-positive-one-year-data-from-phase-ii-altitude-trial-of-abbv-rgx-314-for-treatment-of-diabetic-retinopathy-using-suprachoroidal-delivery-301977598.html
- 4DMT announces first patient enrolled in 4D-150 phase 2 SPECTRA clinical trial in DME, and expansion of 4D-150 phase 2 stage in PRISM clinical trial in wet AMD. News release. September 7, 2023. Accessed April 19, 2024. https://4dmt.gcs-web.com/news-releases/news-release-details/4dmt-announces-first-patient-enrolled-4d-150-phase-2-spectra.










