The need for reliable endpoints for use in diabetic retinopathy (DR) clinical trials was recognized decades ago.1 The Klein Scale from the Wisconsin Epidemiologic Study of DR2,3 was developed to characterize DR severity in a population of diabetic patients surveyed over an extended period of time. These data show relatively high 25-year cumulative rates of progression of DR and incidence of proliferative DR (PDR).3 Klein’s binocular scale used the maximum grade in any of the 7 standard photographic fields to determine Diabetic Retinopathy Severity Scale (DRSS) retinopathy levels (Table 1), and then concatenated the levels from the 2 eyes into 15 steps from mildest (10/10) to most severe (60+/60+), giving the higher level greater weight. In practice, the incidence of vision impairment in patients with minimal to mild DR is quite low,4 and an improved scale was needed to identify eyes at risk for progression. Data from Klein’s epidemiological studies,2 along with the natural history of different levels of retinopathy in the treatment deferred arm of the Early Treatment Diabetic Retinopathy Study (ETDRS), helped to inform how many steps of progression on the DRSS was meaningful as a clinical trial endpoint in strongly predicting the progression to PDR and sight-threatening disease over specific periods of time, including 1-year, 3-year, and 5-year visits.5

The Gold Standard Measure
The Early Treatment Diabetic Retinopathy Study (ETDRS) was originally published in 1985.6 The ETDRS DRSS was developed from the modified Airlie House classification of diabetic retinopathy for use in the pivotal ETDRS study and is considered the gold standard for clinical research purposes.7 The DRSS ranges from 10 (no retinopathy) to 85 (advanced PDR), with 61 serving as the cut-off for mild PDR (Table 1).7
The ETDRS criteria have been used for evaluating progression in numerous landmark studies in DR and AMD including the following:
- The Diabetic Retinopathy Study8;
- Registration studies for ranibizumab9 and aflibercept10 in AMD (safety and efficacy);
- DRCR Network Protocol S (compared PRP to intravitreal ranibizumab 0.5 mg given monthly through 12 weeks, or 1 triamcinolone acetonide 4 mg injection, or sham for eyes with DME and severe nonproliferative DR (NPDR) or PDR11;
- CLARITY (assessed intravitreal aflibercept for previously untreated or post‒laser treated active proliferative diabetic retinopathy)12;
- PANORAMA aflibercept study (targeted patients with DRSS 47 and 53 without DME and BCVA of 20/40 or better)13; and
- Protocol W (moderate to severe NPDR (DRSS 47 to 53) treated with intravitreal aflibercept compared with sham.
In each of these landmark studies evaluating treatment effect, the ETDRS classification scale was applied to the study eye because the intraocular treatments were administered locally. Although the ETDRS classification scale has demonstrated value in assessing DR, this scale has noteworthy reported limitations and criticisms, including the following:4,15
- ETDRS fundus photography typically only covers about 30% of the entire retinal surface14;
- The scale is not well suited to document worsening or improvement of retinal neovascularization in eyes with PDR15;
- It does not address regression of DR severity in the treatment setting15;
- It is directly tied to visual outcomes other than those based on best-corrected central visual acuity15;
- It does not adequately incorporate severity stages for DME that are currently being used to drive care and evaluation of eyes with DME15;
- After anti-VEGF treatment, the DRSS may not adequately reflect the status of the underlying disease16; and
- It has been shown that the fundus appearance (the DRSS staging) can improve after treatment without associated retinal reperfusion.17
Some investigators have noted that an eye that is “natively” low on the DRSS scale responds differently to treatment from an eye that has been treated with anti-VEGF at higher levels and induced to achieve a lower DRSS score.16,18 For instance, an eye with a DRSS score of 43 that is naïve to treatment may not behave the same as one with a score of 53 that then improved to 43 with treatment. Developed before the advent of anti-VEGF therapy, the DRSS was not designed to determine that these eyes may behave differently. If the vascular bed (using ultrawidefield fluorescein angiography [UWF FA]) of these 2 eyes is different, the disease course and response to treatment of the 2 eyes may also be different.16 Goldberg and colleagues have shown that anti-VEGF–induced improvements in DRSS appear more volatile than those from untreated eyes at the same level on the scale.18 These realities in the era of anti-VEGF therapy underscore the need for alternative grading systems.
New Technologies and Agents
One of the recent innovations that is addressing some of these shortcomings and is enhancing the clinician’s ability to identify eyes at risk of DR progression is UWF imaging.19 Ultrawidefield imaging can now capture >80% of the retinal surface, allowing the identification of predominantly peripheral lesions (PPLs).14 Using a standard grading protocol, the evaluation of UWF FA for the extent of retinal nonperfusion area and nonperfusion index has demonstrated reproducibility.19 The presence and extent of PPLs have been associated with increased risks of DR progression.19 Identification of PPLs using UWF FA may indicate the extent of nonperfusion and ischemia and may serve as a prognostic indicator of increased risk of progression. In addition, a prospective, multicenter, longitudinal observational study demonstrated that the presence of PPLs on UWF FA (eg, hemorrhages and microaneurysms) peripheral to the standard 7 fields on ETDRS fundus photography was associated with greater risk of DR disease worsening over 4 years, independent of baseline DRSS score.20
Treatment guidelines for DR remain largely reactive, with treatments being implemented once progression to PDR or vision loss is observed. Prevention is typically limited to maintaining glycemic and blood pressure control, as well as advocating a healthy diet and lifestyle.21,22 More proactive interventions for NPDR are needed.
New systemic agents are being evaluated for DR. To assess these agents, an updated scale that is suitable for evaluating both eyes is preferable. The ACCORD study included a subset of patients with type 2 diabetes who were at high risk for cardiovascular disease to receive either intensive or standard treatment for glycemia and also for dyslipidemia (fenofibrate plus simvastatin or placebo plus simvastatin) or for systolic blood-pressure control.23 Eyes were evaluated for the effects of these interventions at 4 years on the progression of DR by 3 or more steps or the development of DR requiring laser photocoagulation or vitrectomy.23 ACCORD used an expanded 17-step scale with an abbreviated and modified version of the person ETDRS final retinopathy severity scale (10/10 to 71+/71+), which combines the severity levels from both eyes for each person (Table 2). The extended upper levels of the ACCORD binocular person scale obviated the ceiling effect that was present with the binocular Klein scale, which topped off at level 15 (60+/60+), making it insensitive to DR progression beyond that level.
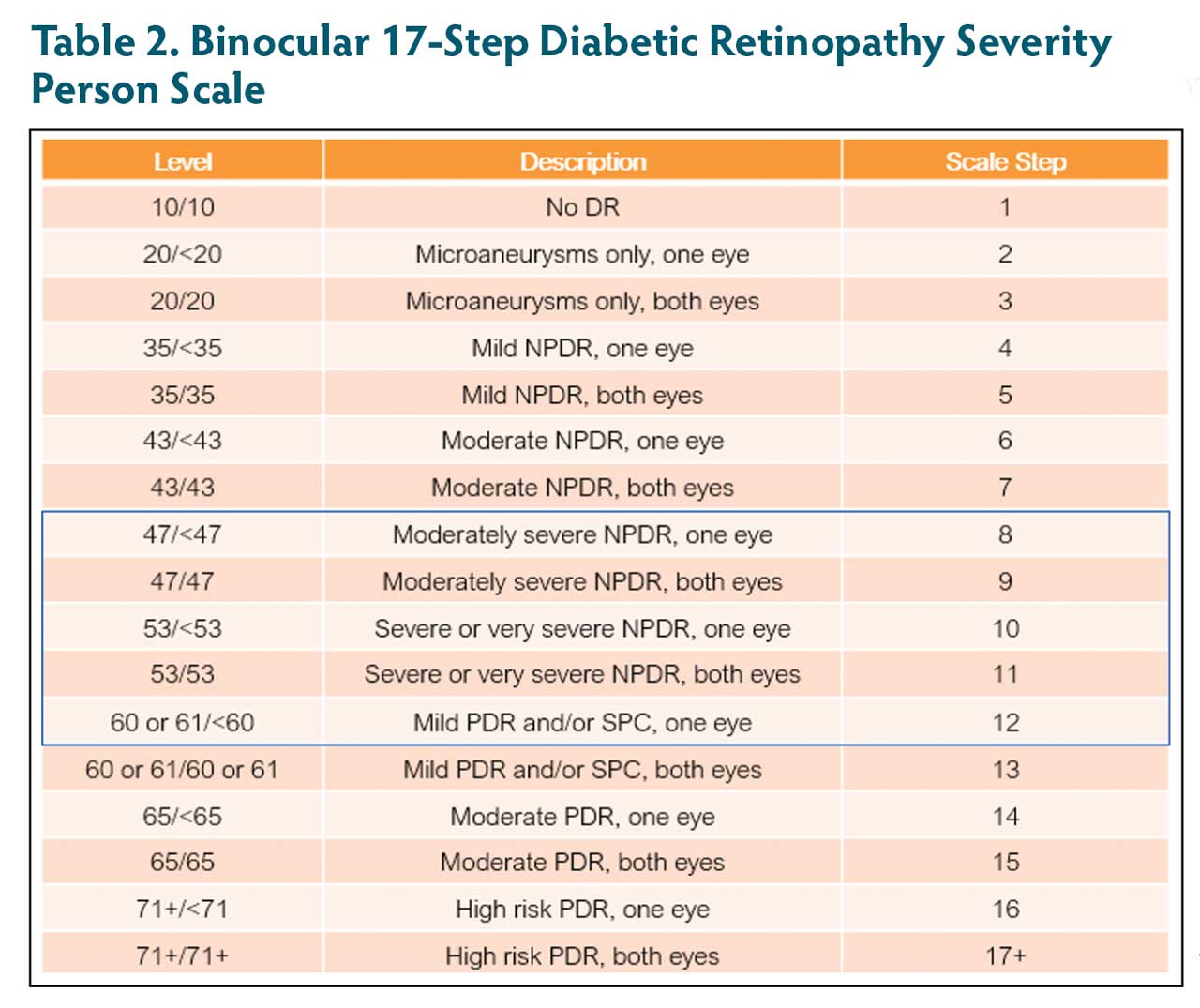
With the person scale shown in Table 2, at baseline and at the final outcome time point, the status of the worse eye is weighted to determine the scale step instead of using simple summation. A 3-step change on this scale may be considered a clinically meaningful clinical study endpoint for registration trials. For example:
- A baseline 47/43 (step 8)
- Final 47/47 (step 9; 1-step change)
- Final 53/43 (step 10; 2-step change)
- Final 61/43 (step 12; 4-step change)
- Final 61/53 (step 12; 4-step change)
Another example is a patient who starts at 47/43 who worsens from 47 to 61 in the worse eye and improves in the better eye to 35, also has a 4-step change (step 8 to step 12). This scale is being adopted for studies evaluating systemic drugs for DR.
Recent studies with systemic agents targeted at DR that have adopted the person scale include the ZETA-1 and CANBERRA studies. The ZETA-1 study was conducted to assess the efficacy of oral APX3330 to improve the ETDRS DRSS in participants with moderately severe to severe NPDR or mild PDR.24 This study began as a monocular study using greater or equal to 2 steps of either improvement or preventing progression in the study eye as its primary endpoint. However, after receiving FDA guidance, the ZETA-1 was evaluated using the 17-step binocular person scale, using 3 or more step improvement or prevention of worsening, which will be a registration endpoint for future studies. The CANBERRA study was a 48-week trial designed to evaluate the safety, tolerability, and effect of oral administration of RG7774 on the severity of DR in participants with moderately severe to severe NPDR and good vision.25
A new generation of DR screening innovations may lead to additional clinical endpoints that could be adopted for clinical research.1 These include the following:
- Scanning laser ophthalmoscope–based nonmydriatic UWF with a field capture of as much as 200°19
- Adaptive optics
- Doppler: blood flow, oxygenation,
perfusion - Stroboscopic fundus cameras
- Optical coherence angiography
- Retinal oximetry
- Retinal metabolic imaging
Machine learning (ML) algorithms have the potential to identify eyes early in the DR disease process.A recent study used UWF images from patients with mild or moderate NPDR with 3 years of longitudinal follow-up retinal imaging or evidence of progression within 3 years to develop automated ML models for predicting DR progression.26 In the validation set, 6 of 9 eyes (75%) with mild NPDR and 35 of 41 eyes (85%) with moderate NPDR that progressed ≥2 steps were identified. All 4 eyes with mild NPDR that progressed at 6 months and 1 year were identified, as were 8 of 9 (89%) and 17 of 20 (85%) of eyes with moderate NPDR that progressed at 6 months and 1 year, respectively. The study demonstrated the accuracy and feasibility of using automated ML models in conjunction with UWF images for identifying eyes at high risk of DR progression. Prospective studies and regulatory approval will be required before these models can be used widely by physicians in the clinical setting. These results highlight increasing accessibility to ML applications, which can address an unmet clinical need for rapid accurate screening. In the future, these algorithms may be able to identify patients at the highest short-term risk for progression, with the goal of reducing costs and improving vision outcomes in DR.
Conclusion
Considerable advancements, occurring over the past several decades, contributed to the refinement and evolution of DR clinical endpoints. Ultrawidefield imaging is expanding the field of view and increasing the odds of identifying those at risk for DR. A 17-step binocular DR severity scale has been developed that can be harnessed in clinical trials of new systemic agents designed to prevent and slow the progression of DR. ML algorithms have the potential to automate the identification of eyes at risk for DR progression, reducing costs and improving visual outcomes. These and other innovations may serve to refine the clinical endpoints for future clinical studies in DR. RP
Editor's note: Hear discussion of this article at retinapodcast.com.
References
1. Nair P, Aiello LP, Gardner TW, Jampol LM, Ferris FL, III. Report from the NEI/FDA Diabetic Retinopathy Clinical Trial Design and Endpoints Workshop. Invest Ophthalmol Vis Sci. 2016;57(13):5127-5142. doi:10.1167/iovs.16-20356
2. Klein R, Klein BE, Moss SE. How many steps of progression of diabetic retinopathy are meaningful? The Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2001;119(4):547-553. doi:10.1001/archopht.119.4.547
3. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503. doi:10.1016/j.ophtha.2008.10.016
4. Chew EY. Surrogate outcomes for clinical trials: use with caution. Arch Ophthalmol. 2001;119(4):576-578. doi:10.1001/archopht.119.4.576
5. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766-785. https://www.ncbi.nlm.nih.gov/pubmed/2062512.
6. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-1806. https://www.ncbi.nlm.nih.gov/pubmed/2866759.
7. Yonekawa Y, Modi YS, Kim LA, Skondra D, Kim JE, Wykoff CC. American Society of Retina Specialists clinical practice guidelines on the management of nonproliferative and proliferative diabetic retinopathy without diabetic macular edema. J Vitreoretin Dis. 2020;4(2):125-135. doi:10.1177/2474126419893829
8. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583-600. https://www.ncbi.nlm.nih.gov/pubmed/7196564.
9. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi:10.1056/NEJMoa054481
10. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. doi:10.1016/j.ophtha.2012.09.006
11. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi:10.1001/jama.2015.15217
12. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept vs panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi:10.1016/S0140-6736(17)31193-5
13. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946-955. doi:10.1001/jamaophthalmol.2021.2809
14. Solomon SD, Goldberg MF. ETDRS grading of diabetic retinopathy: still the gold standard? Ophthalmic Res. 2019;62(4):190-195. doi:10.1159/000501372
15. Sun JK, Aiello LP, Abramoff MD, et al. Updating the staging system for diabetic retinal disease. Ophthalmology. 2021;128(4):490-493. doi:10.1016/j.ophtha.2020.10.008
16. Jampol LM, Tadayoni R, Ip M. Need for a new classification of diabetic retinopathy. Retina. 2021;41(3):459-460. doi:10.1097/IAE.0000000000003070
17. Wykoff CC, Nittala MG, Zhou B, et al. Intravitreal aflibercept for retinal nonperfusion in proliferative diabetic retinopathy: outcomes from the randomized RECOVERY trial. Ophthalmol Retina. 2019;3(12):1076-1086. doi:10.1016/j.oret.2019.07.011
18. Goldberg RA, Hill L, Davis T, Stoilov I. Effect of less aggressive treatment on diabetic retinopathy severity scale scores: analyses of the RIDE and RISE open-label extension. BMJ Open Ophthalmol. 2022;7(1). doi:10.1136/bmjophth-2022-001007
19. Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122(12):2465-2472. doi:10.1016/j.ophtha.2015.07.034
20. Marcus DM, Silva PS, Liu D, et al. Association of predominantly peripheral lesions on ultra-widefield imaging and the risk of diabetic retinopathy worsening over time [published correction appears in JAMA Ophthalmol. 2023;141(1):104]. JAMA Ophthalmol. 2022;140(10):946-954. doi:10.1001/jamaophthalmol.2022.3131
21. American Academy of Ophthalmology. Diabetic retinopathy preferred practice pattern 2019. Accessed March 1, 2024. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp
22. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412-418. doi:10.2337/dc16-2641
23. The Accord Study Group and Accord Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-244. doi:10.1056/NEJMoa1001288
24. Ocuphire Pharma, Inc. Study of the safety and efficacy of APX3330 in diabetic retinopathy (ZETA-1). Clinicaltrials.gov identifier: NCT04692688. Updated February 27, 2023. Accessed March 4, 2024. https://clinicaltrials.gov/study/NCT04692688
25. Hoffmann-La Roche. A study to investigate the efficacy and safety of RG7774 in patients with diabetes mellitus type 1 or type 2 with treatment-naive diabetic retinopathy (CANBERRA). Clinicaltrials.gov identifier: NCT04265261. Updated January 17, 2024. Accessed March 4, 2024. https://clinicaltrials.gov/study/NCT04265261
26. Silva PS, Zhang D, Jacoba CMP, et al. Automated machine learning for predicting diabetic retinopathy progression from ultra-widefield retinal images. JAMA Ophthalmol. 2024. doi:10.1001/jamaophthalmol.2023.6318













