Diabetic retinopathy (DR), and its most vision-threatening complication diabetic macular edema (DME), is a leading cause of vision loss and blindness in the working-age population.1 Early detection and timely intervention are crucial to prevent irreversible damage. To this end, recent advancements in ocular imaging modalities have revolutionized the diagnosis and management of diabetic eye disease. But interestingly, the retinal treatment space is progressing so rapidly, and demonstrating so much promise, that the technologies we use to assess the need for treatment are being evaluated through a new lens.
As we move toward a future in which we can treat diabetic eye disease sooner, less invasively, and with greater precision, researchers are scrutinizing the structural and functional tools that we use in practice and that are used as endpoints in clinical trials for these emerging agents. This article aims to elucidate these research endeavors by providing an overview of several technologies from the perspective of the Early Treatment Diabetic Retinopathy Study (ETDRS) and, more recently, the Mary Tyler Moore (MTM) Vision Initiative’s Diabetic Retinal Disease (DRD) Staging Update Project and Clinical Endpoints Workshop.
Early Treatment Diabetic Retinopathy Study History
The ETDRS was conducted in the 1980s and established standardized methods for grading DR and evaluating treatment efficacy. The ETDRS was an historic study, and its findings still influence the management of DR today. ETDRS demonstrated the benefit of focal macular laser in the treatment of DME, also defining the criteria for treatment of "clinically significant macular edema," which are still used today for determining which patients should be treated with focal laser. The ETDRS study was crucial in determining that panretinal photocoagulation (PRP) reduces the risk of disease progression and vision loss and can be delayed until the development of severe nonproliferative or early proliferative stage retinopathy. ETDRS also concluded that early vitrectomy should be considered for advanced active proliferative DR. Additionally, the ETDRS study established that aspirin has no role in preventing the progression of DR, but that it is not contraindicated and can be used if cardiovascular indications require it.2-6
Mary Tyler Moore Vision Initiative
Diabetes-related vision loss had an enormous impact on Mary Tyler Moore, inspiring her work with the Juvenile Diabetes Research Foundation (JDRF) to help find cures for type 1 diabetes and its complications. Moore’s husband, S. Robert Levine, a Mt. Sinai Medical Center cardiologist, played an integral role in the foundation and, following his wife’s passing, created and chaired the MTM Vision Initiative, the goal of which is to create a world without vision loss from diabetic retinal disease. The MTM Vision Initiative follows a well-defined 3-phase roadmap. The inaugural global workshop was held in 2022. It was attended by clinicians, vision scientists, pharmaceutical and diagnostic device companies, regulatory agencies, and patients and their representatives who worked together to address clinical endpoints.16 In November 2023, the second workshop was held on World Diabetes Day.
The question of diabetes staging is central to the MTM plan because it encapsulates the first challenge that the foundation seeks to overcome. Namely, MTM Vision's DRD staging update project centers on the premise that “you cannot solve a problem you have not defined.”16 In tandem, the MTM Vision Initiative’s Clinical Endpoints Workshop was designed to address a related challenge, which is that “you cannot judge success unless you know what to measure.” The primary purpose of this workshop is to identify appropriate clinical endpoints, which include both structural imaging and functional assessments.16
Structural Imaging Techniques
From an imaging perspective, ETDRS introduced a comprehensive set of standardized retinal photographs that became the gold standard for assessing diabetic eye disease severity and progression. These photographs are still widely used in clinical practice and serve as a benchmark for comparison with newer imaging modalities.7
Fundus Photography
Fundus photography (FP) is a noninvasive imaging technique that captures high-resolution images of the retina. It provides a detailed view of the retinal vasculature, macula, and optic nerve head. The images obtained through fundus photography are used for diagnosing and monitoring DR directly in correlation with the ETDRS recommendations. Several devices utilizing fundus photography have received FDA approval and are commonly used in clinical settings.
Widefield imaging involves capturing images of a larger area of the retina beyond the central macula. Several commercial devices are available that provide a panoramic view of the retina and allow for the detection and monitoring of peripheral retinal changes. Figure 1 demonstrates pseudowidefield color fundus montage images demonstrating severe nonproliferative DR and DME in both eyes of a 68-year-old woman who presented with vision loss.
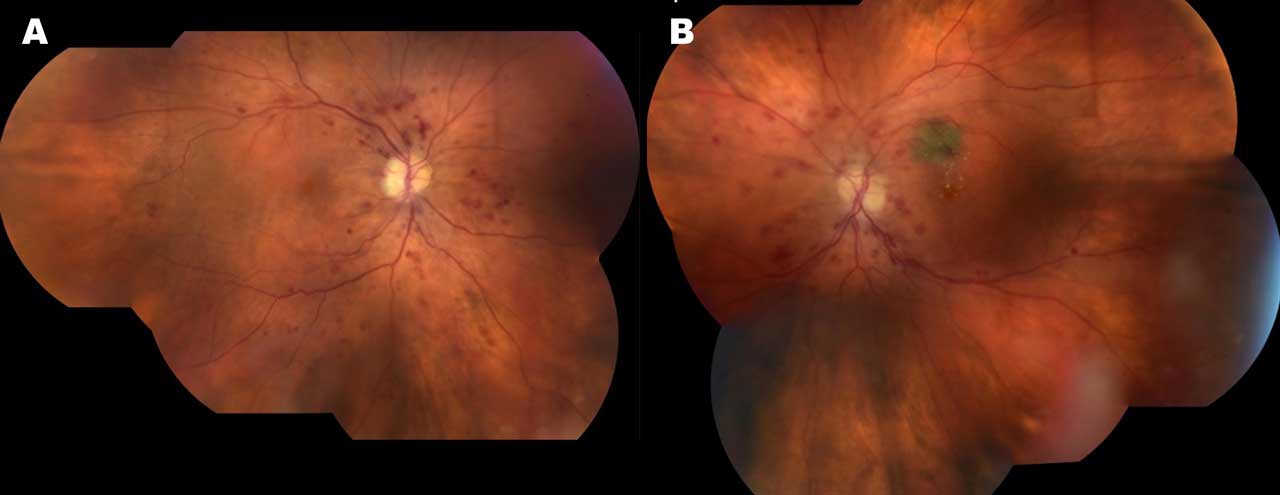
Hirano et al compared the Clarus 500TM (Carl Zeiss Meditec AG) and Optos (Optos PLC) widefield cameras to examine eyes with DR using single images obtained with mydriasis, showing both systems are generally consistent in assessing DR severity.8
Another study compared the effectiveness of widefield imaging using the Clarus fundus camera with traditional slit lamp fundus examination in assessing patients referred from the British National Health Service DR screening program. The results indicated that the Clarus camera provides a wider and clearer image of the retina, offering a more comprehensive view of the peripheral areas, leading to more accurate diagnoses and better management of DR. The authors concluded that the Clarus camera might be considered as a primary tool in DR screening due to its superior imaging capabilities, but also emphasized that the slit lamp examination remains a valuable method in assessing certain specific retinal conditions.9
Widefield imaging has shown promise in identifying subtle DR lesions that may be missed by conventional imaging techniques. Further research is needed to establish its role in routine clinical practice.
Optical Coherence Tomography
Optical coherence tomography (OCT) is a noninvasive imaging technique that uses light waves to create cross-sectional images of the retina. It enables the visualization of retinal layers and the detection of subtle changes in retinal thickness. This modality has emerged as a valuable tool for diagnosing and managing DR and DME, allowing for early disease detection and monitoring treatment response. Various OCT devices have received FDA approval and are widely used by ophthalmologists.
Markan et al explored the use of novel imaging biomarkers in diagnosing and treating DR and DME. The authors discuss key biomarkers specific to spectral domain OCT including disorganization of retinal inner layers (DRIL), hyperreflective foci, and ellipsoid zone (EZ) integrity. The authors also review prior studies concluding that integrity of outer retinal layers including the EZ is a direct indicator of the health of the retinal photoreceptors, and suboptimal visual acuity gains are often seen in DR/DME patients with persistent outer retinal disruption. OCT has universally become a gold standard for diagnosing and treating DME and thus plays a critical role for assessment of any DR patient with vision loss.10
Figures 2 and 3 demonstrate the OCTs of the same patient example who received serial intravitreal injections over 5 months. Besides an overall reduction in the DME-associated retinal thickness, the right eye has a reduction in the quantity of hyperreflective foci present.
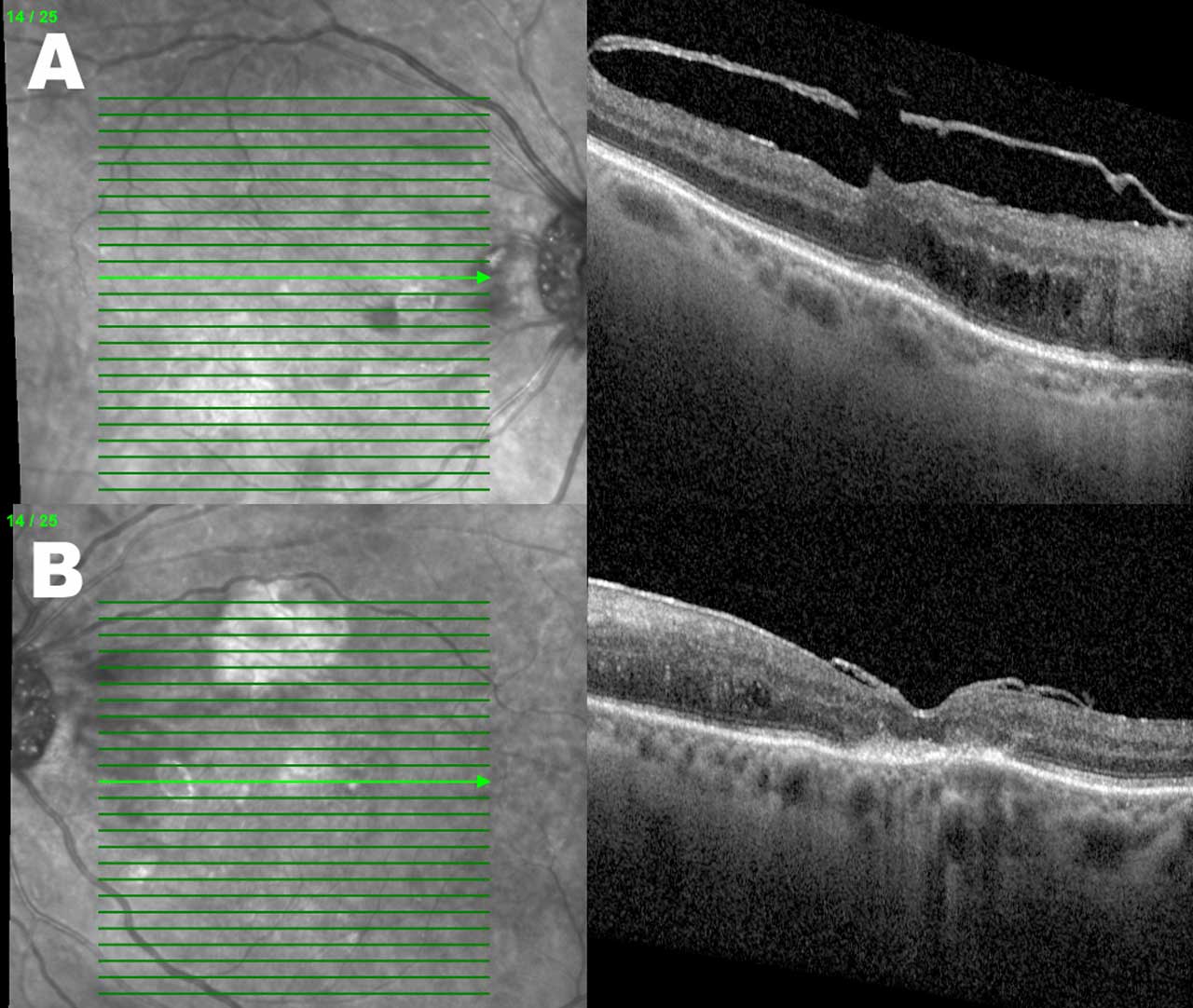
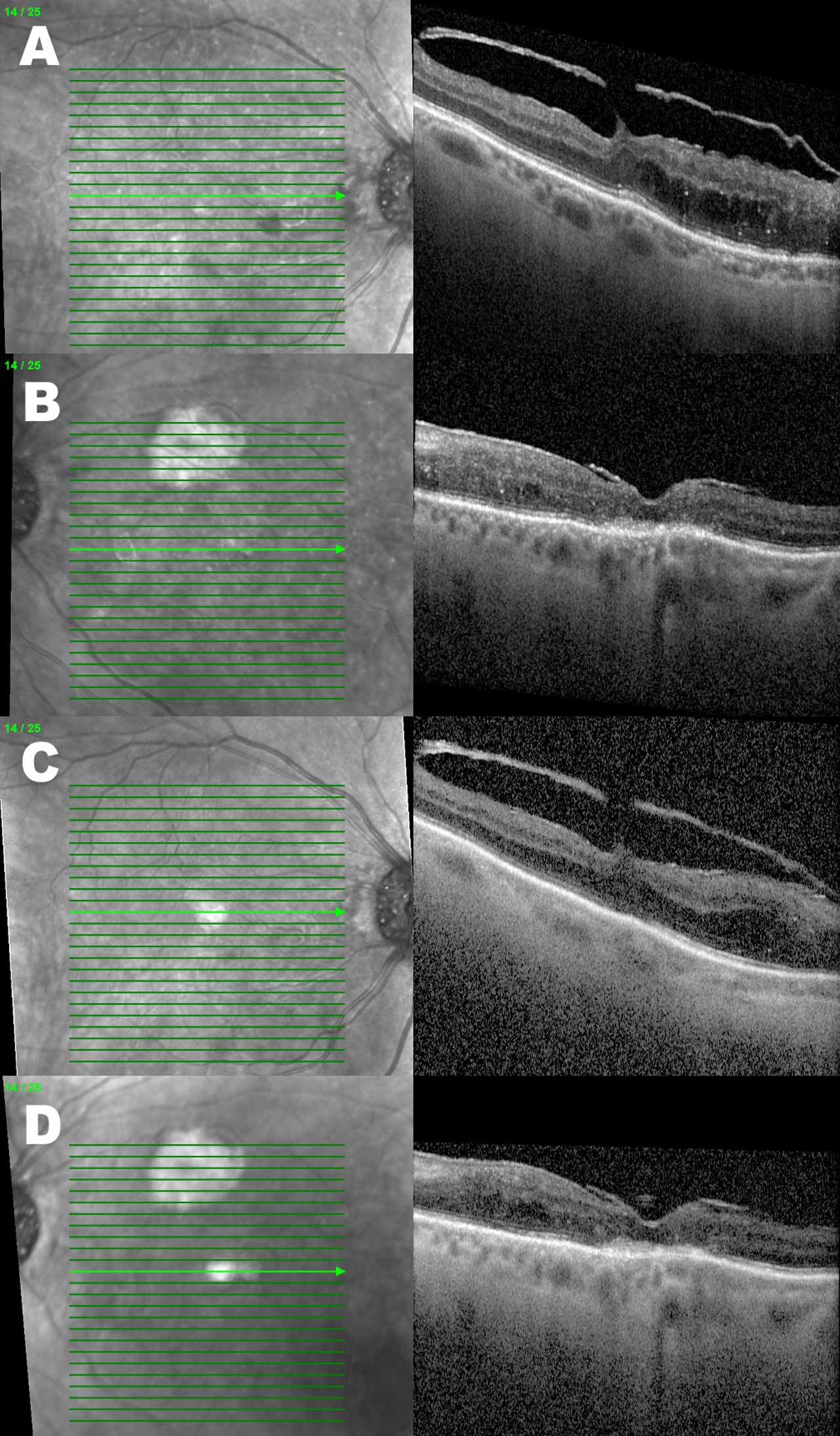
Fluorescein Angiography
Ultrawidefield fluorescein angiography (FA) has an essential role in diagnosing and managing DR, because it provides detailed imaging of the retina, thereby enabling more accurate diagnoses. This diagnostic procedure involves injecting a fluorescent dye into the bloodstream and capturing sequential images of the retina as the dye circulates. It helps identify areas of vascular leakage and abnormal blood vessel growth, aiding in the diagnosis and management of DR. As demonstrated by ETDRS, FA assists in determining the likelihood of progression to proliferative retinopathy by enabling visibility of fluorescein leakage, capillary loss and dilatation, and arteriolar abnormalities.11 Researchers have concluded that this technology can significantly improve the management and treatment of DR.12
Many studies have supported the link between DME and peripheral ischemia by quantitatively assessing capillary nonperfusion, generating an ischemic index (ISI) representing the percentage of ischemia over the total retina area.12 Optos imaging, while useful, introduces peripheral distortion that impacts ISI calculation.12 To address this, researchers have proposed a corrected ISI using stereographic projection software, which was found to correlate strongly with the uncorrected index.17
A positive correlation between ISI and DME also has been noted, with peripheral ischemia being an independent risk factor for DME development.18 Furthermore, researchers believe that higher DR severity and ISI are associated with worse recalcitrant DME.19 A positive correlation between peripheral and diabetic macular ischemia suggests a common pathogenesis involving capillary nonperfusion.20
Importantly, although ultrawidefield angiography is primarily a diagnostic tool, it may also have a role in treating DR. Given the side effects associated with PRP, such as visual acuity reduction and DME onset, targeted retinal photocoagulation (TRP) of ischemic areas has been proposed. Targeted retinal photocoagulation likely results in neovascular regression without PRP-related side effects.21,22 In fact, a pilot randomized study showed that TRP was as effective as standard intensity (SI) PRP in inducing neovascular regression but with a greater reduction in central macular thickness (CMT).23 However, it bears noting that, although widely used, FA has certain limitations, such as the need for intravenous dye injection and potential allergic reactions.
Figure 4 shows pseudowidefield fluorescein angiogram montage images demonstrating nonperfusion, ischemic changes, and DME leakage in both eyes and leakage due to neovascularization elsewhere in the left eye of the same patient example.
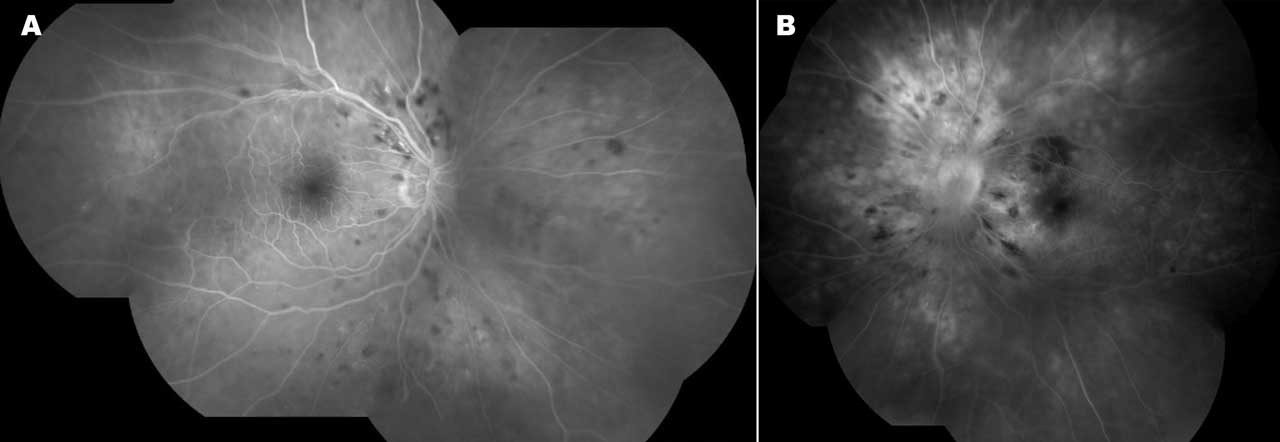
Adaptive Optics Imaging
Adaptive optics imaging (AOI) is another cutting-edge technology that enables high-resolution imaging of the retina, in this case at the cellular level. It corrects for optical aberrations in the eye to obtain detailed images of individual retinal cells. A prospective case series studying the correlation between FA and AO in DR eyes found a significant association between these 2 methods, potentially offering a more integrated and enhanced approach toward diagnosing and treating DR.13 This technique holds great promise for early detection and monitoring of DR, because it can visualize microscopic changes in retinal structure. However, its widespread use is limited by its high cost and complexity.
Functional Testing
Although the role of structure cannot be understated, functional measures are arguably too often overlooked. Beyond subjective measures of function, such as visual acuity and visual fields, objective measures of retinal cell functioning are emerging as key markers of disease progression, with striking implications in terms of how we stage diabetic retinal disease. Currently, the functional endpoint that is being explored for the purposes of staging and endpoint validation is electroretinography (ERG).13
Electroretinography
Electroretinography (ERG) is a technique that measures the electrical responses of the retina to light stimulation. It provides valuable information about retinal function and can detect early functional changes associated with DR. A recent publication explored potential of ERG in detecting early retinal dysfunction in diabetic patients, even before structural changes are evident. The findings suggested that ERG could be a valuable tool for early detection and monitoring of DR.14
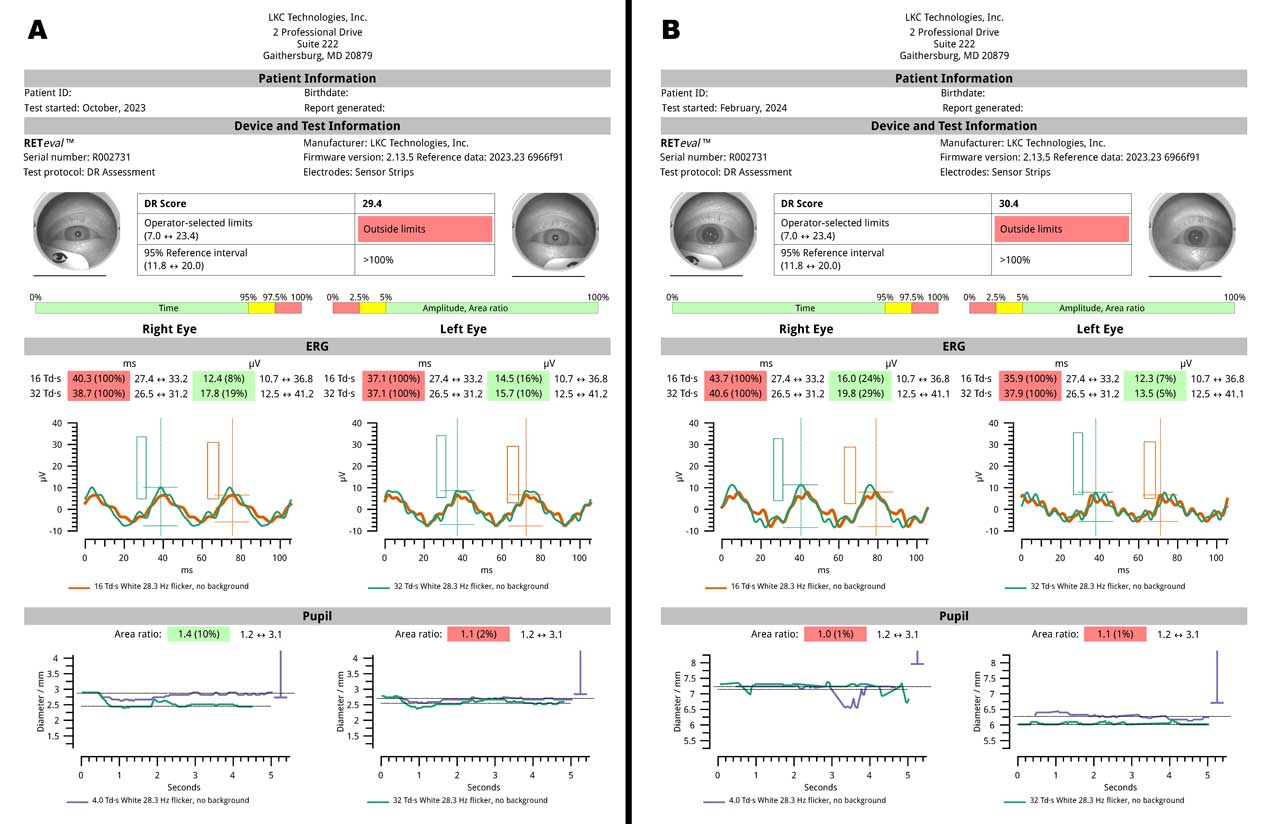
Brigell et al conducted a cross-sectional study using a commercially available ERG device (RETeval; LKC Technologies) analyzing charts of 252 patients with diabetes up to 3 years after initial ETDRS 7-field stereo fundus imaging and evaluation of physiologic measures of retinal function (ERG and pupillary light reflex).15 Predictive performance of the functional measures, structural measures, and their combination were assessed in terms of subsequent need for medical or surgical ocular interventions required to treat vision-threatening complications including intravitreal injections, laser treatment, and vitrectomy. Results were used to develop a risk value (DR score) that increased and was closely correlated with worsening DR severity based on double-graded ETDRS fundus photos of the tested patients by a dedicated reading center. The DR score was found to indicate the highest risk of requiring treatment intervention, with patients having scores ≥23.5 being 11 times more likely to have a future intervention than patients having scores <23.5. The authors concluded that this integrated approach helps capture the disease's full extent, which may be overlooked when only 1 type of test is used and can enhance patient management, allowing for targeted treatment strategies and better prediction of disease progression.
Figure 5 demonstrates serial RETeval ERGs 5 months apart in the same patient. Despite several initial injections of off-label bevacizumab and reductions of central retinal thickness seen on OCT in the right eye, with more modest improvements in the left eye, the DR score worsened from 29.4 to 30.4, prompting the clinical decision to switch to on-label aflibercept intravitreal injections in both eyes for subsequent treatments to try to improve the clinical response.
Conclusion
Advancements in imaging and functional testing have significantly improved the diagnosis and management of diabetic eye disease, yet our current staging system is based on fundus photos, which falls short of providing the depth of information that we can glean when applying newer modalities.14 Although it has been the standard since the 1980s, ETDRS fails to capture the totality of retinal damage in diabetic retinal disease because it is limited exclusively to identifiable microvascular structural lesions.14
The Mary Tyler Moore Vision Initiative’s DRD Staging Update Project is a next step in the evolution of a clinically useful system for applying new modalities.14 This continued research will pave the way for more precise and personalized approaches to managing this sight-threatening condition.
Editor’s note: Listen to discussion of this article on the Retina Podcast at retinapodcast.com.
References
1. Keenum Z, McGwin G Jr, Witherspoon CD, Haller JA, Clark ME, Owsley C. Patients' adherence to recommended follow-up eye care after diabetic retinopathy screening in a publicly funded county clinic and factors associated with follow-up eye care use. JAMA Ophthalmol. 2016;134(11):1221-1228. doi:10.1001/jamaophthalmol.2016.3081
2. Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995;113(9):1144-1155.
3. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(5 Suppl):766-785.
4. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786-806.
5. Flynn HW Jr, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL 3rd. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992;99(9):1351-1357. doi:10.1016/s0161-6420(92)31779-8
6. Early Treatment Diabetic Retinopathy Study Research Group. Effects of aspirin treatment on diabetic retinopathy. ETDRS report number 8. Ophthalmology. 1991;98(5 Suppl):757-765.
7. Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(5 Suppl):823-833.
8. Hirano T, Imai A, Kasamatsu H, Kakihara S, Toriyama Y, Murata T. Assessment of diabetic retinopathy using 2 ultra-wide-field fundus imaging systems, the Clarus and Optos systems. BMC Ophthalmol. 2018;18(1):332. doi:10.1186/s12886-018-1011-z
9. Lim WS, Grimaldi G, Nicholson L, Basheer K, Rajendram R. Widefield imaging with Clarus fundus camera vs slit lamp fundus examination in assessing patients referred from the National Health Service diabetic retinopathy screening programme. Eye (Lond). 2021;35(1):299-306. doi:10.1038/s41433-020-01218-x
10. Markan A, Agarwal A, Arora A, Bazgain K, Rana V, Gupta V. Novel imaging biomarkers in diabetic retinopathy and diabetic macular edema. Ther Adv Ophthalmol. 2020;12:2515841420950513. doi:10.1177/2515841420950513
11. Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS report number 13. Ophthalmology. 1991;98(5 Suppl):834-840.
12. Rabiolo A, Parravano M, Querques L, et al. Ultra-wide-field fluorescein angiography in diabetic retinopathy: a narrative review. Clin Ophthalmol. 2017;11:803-807. doi:10.2147/OPTH.S133637
13. Huang RS, Mihalache A, Popovic MM, et al. Association of intravenous fluorescein angiography and adaptive optics imaging in diabetic retinopathy: a prospective case series. Retina. 2024;44(4):689-699. doi:10.1097/IAE.0000000000004012
14. Channa R, Wolf RM, Simo R, et al. A new approach to staging diabetic eye disease: staging of diabetic retinal neurodegeneration and diabetic macular edema. Ophthalmol Sci. 2023;4(3):100420. doi:10.1016/j.xops.2023.100420
15. Brigell MG, Chiang B, Maa AY, Davis CQ. Enhancing risk assessment in patients with diabetic retinopathy by combining measures of retinal function and structure. Transl Vis Sci Technol. 2020;9(9):40. doi:10.1167/tvst.9.9.40
16. Levine SR, Myers MG, Barunas R, et al. Report from the 2022 Mary Tyler Moore Vision Initiative Diabetic Retinal Disease Clinical Endpoints Workshop. Transl Vis Sci Technol. 2023;12(11):33. doi:10.1167/tvst.12.11.33
17. Tan CS, Chew MC, van Hemert J, Singer MA, Bell D, Sadda SR. Measuring the precise area of peripheral retinal non-perfusion using ultra-widefield imaging and its correlation with the ischaemic index. Br J Ophthalmol. 2016;100(2):235-239. doi:10.1136/bjophthalmol-2015-306652
18. Wessel MM, Nair N, Aaker GD, Ehrlich JR, D'Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-698. doi:10.1136/bjophthalmol-2011-300774
19. Patel RD, Messner LV, Teitelbaum B, Michel KA, Hariprasad SM. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155(6):1038-1044.e2. doi:10.1016/j.ajo.2013.01.007
20. Sim DA, Keane PA, Rajendram R, et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014;158(1):144-153.e1. doi:10.1016/j.ajo.2014.03.009
21. Reddy S, Hu A, Schwartz SD. Ultra-widefield fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9-14. doi:10.1080/08820530802519899
22. Muqit MM, Marcellino GR, Henson DB, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91(3):251-258. doi:10.1111/j.1755-3768.2011.02307.x
23. Muqit MM, Young LB, McKenzie R, et al. Pilot randomised clinical trial of Pascal TargETEd Retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. Br J Ophthalmol. 2013;97(2):220-227. doi:10.1136/bjophthalmol-2012-302189









