Although macular changes are the hallmark of age-related macular degeneration (AMD), in the last decade, it has been noted that peripheral changes have a higher prevalence in AMD patients compared to the general population.1,2 This has led to an increased interest in exploring their significance.
The Peripheral Retina
There is currently no consensus on the definition of what encompasses the peripheral retina,3 but in general “peripheral retina” refers to the areas of the retina found outside of the macula and beyond the vascular arcades. For example, some studies defined the periphery based on degrees — outside of the 50° to 60° central area,4 or outside of the central 30° centered on the fovea.5
The peripheral retina contributes to our ability to navigate the world, detect motion, and maintain orientation. Recent advancements in imaging technology have revolutionized our ability to assess the peripheral retina, and increased awareness of peripheral alterations in various diseases. Namely, ultrawidefield (UWF) imaging has emerged as a valuable tool in capturing peripheral retinal abnormalities. Unlike conventional imaging, UWF provides a panoramic view of up to 200°, allowing for a comprehensive assessment of the peripheral regions. The International Widefield Imaging Study Group defined UWF imaging to describe images showing the retina anterior to the vortex vein ampullae in all 4 quadrants.6
Using UWF imaging, researchers have recently proposed new definitions of retinal zones to be considered in the assessment of patients with AMD. In addition to the macular zones 1-3 proposed by the international classification and age-related eye disease classification, Lengyel et al7 defined a zone 4 as the mid periphery (with a diameter of 11,000 μm) and zone 5 as the far periphery (all areas outside zone 4). Similarly, the OPERA8 (Peripheral Retinal Changes Associated with Age-related Macular Degeneration in the Age-related Eye Disease Study 2) 2017 study defined zone 1 as correspondent to the posterior pole (5.4 mm radius/3 disc diameters), zone 2 to the midperiphery (extending from the edge of zone 1 anteriorly with a radius of 16.2 mm or 9 disc diameters, overlapping the vortex veins), and zone 3 as the region anterior to zone 2. In this landmark study, the authors also developed a standardized grid to facilitate the definition and quantification of peripheral retinal changes, enabling more consistent documentation and comparisons across studies.
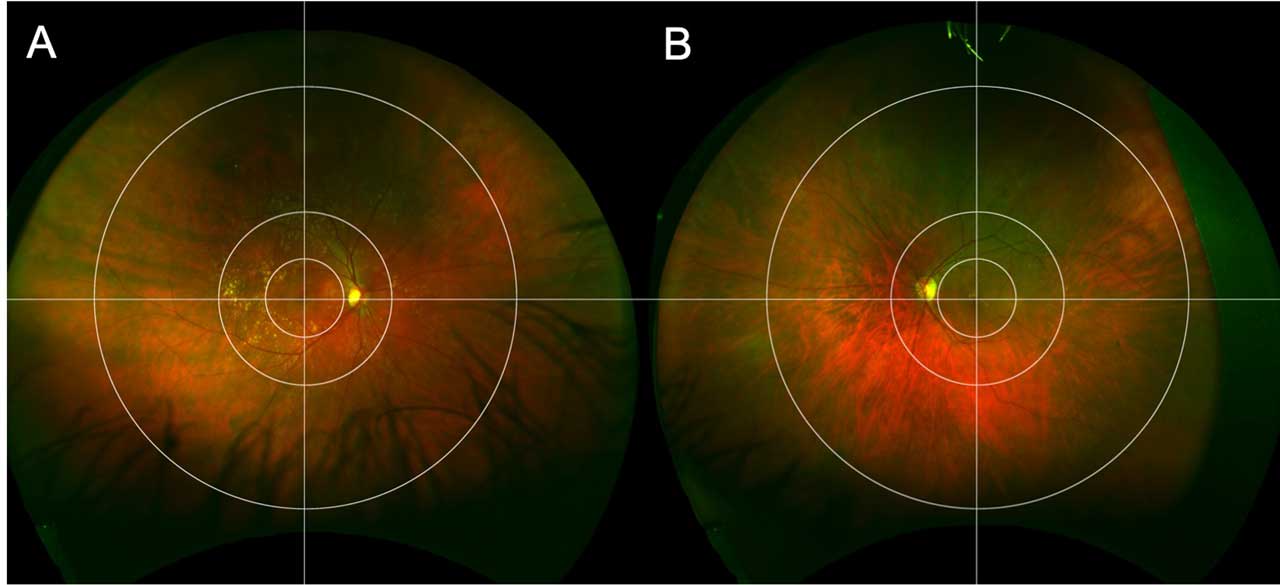
The Boston grid (Figure 1) is an alternative system,9 with the aim to specifically focus on peripheral and posterior pole retinal abnormalities in AMD. This grid, which is based on image size rather than dimensions in the eye, includes 3 concentric zones centered on the fovea with perpendicular crosshairs centered at the fovea as well. The first circle surrounds the macula within the vascular arcades, with a radius of 2.9 mm, and is based on the Early Treatment of Diabetic Retinopathy Study (ETDRS) grid. The second circle encompasses the perimacular area, including both temporal arcades, with a radius of 6.5 mm. The third peripheral circle separates the midperipheral area from the far periphery, with a radius of 16 mm. These, among other grids, have been used in the study of peripheral changes in AMD.
Peripheral Retinal Changes in AMD
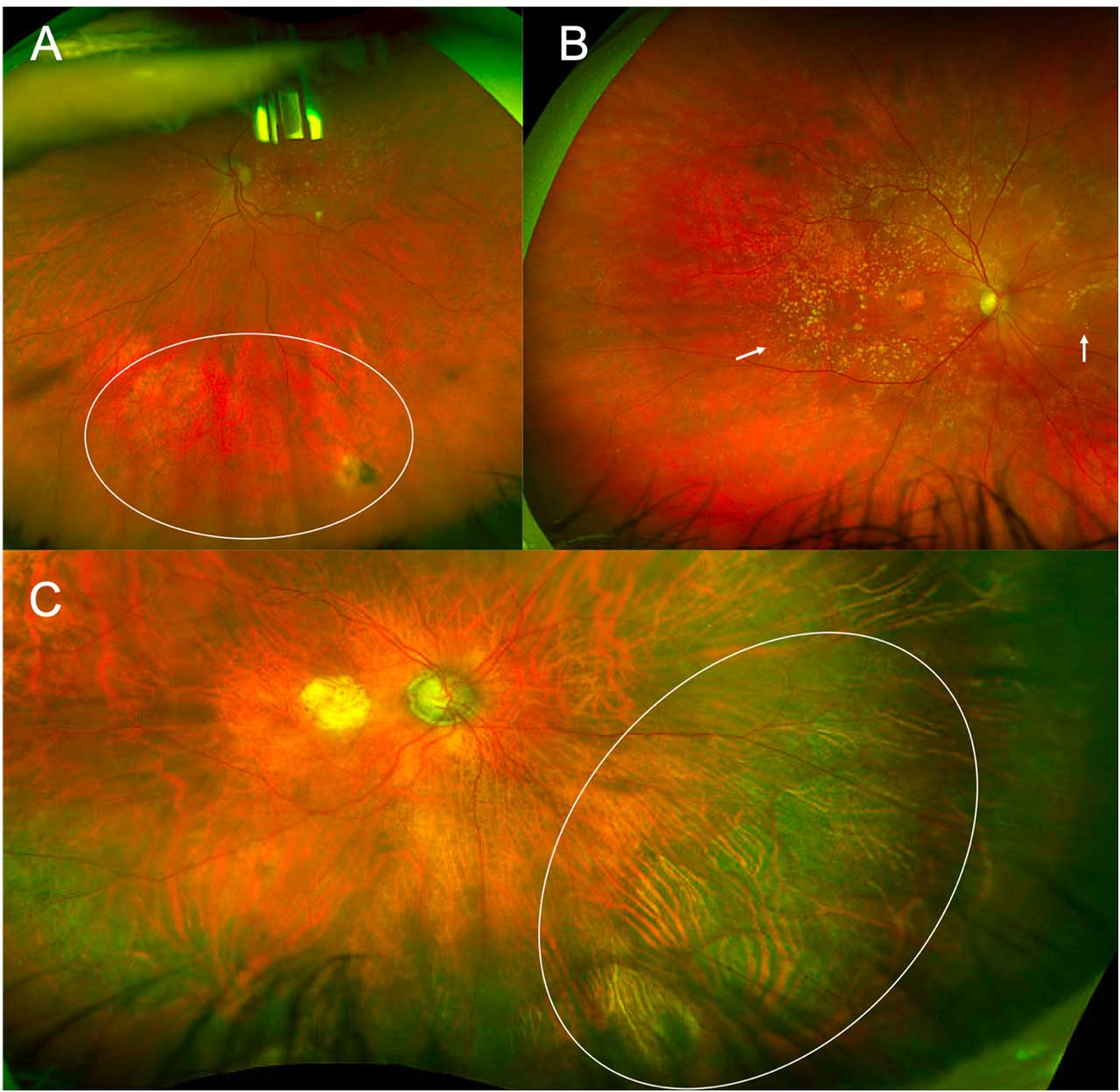
Recent data suggest that peripheral changes are more commonly seen in patients with AMD than controls. For example, the landmark 2017 OPERA study8 reported that drusen were more commonly seen in the periphery in patients with AMD (up to 97%) compared to controls (48%). Similar findings were reported for reticular pigmentary changes (Figure 2A), which were observed in the midperiphery of 48% of the eyes with AMD vs 16% of the controls. In addition to classic drusen and reticular pigmentary changes, other peripheral changes seen in patients with AMD include subretinal drusenoid deposits, hypopigmented and/or hyperpigmented areas and focal areas of chorioretinal atrophy (Figure 2). Extramacular peripheral choroidal neovascularization has also been described in 5% of the cases.8 This phenomenon, called peripheral exudative hemorrhagic chorioretinopathy (PEHCR) may be more common in patients with AMD or may be related to similar pathophysiological underpinnings,10 and associations between PEHCR and drusenoid deposits in the macula have been reported.11
Interestingly, various studies have also shown an association between specific AMD risk single-nucleotide polymorphisms (SNPs) and peripheral abnormalities in AMD. Shuler et al12 demonstrated an association between peripheral reticular pigmentary changes and the complement factor H (CFH) Y420H polymorphism, whereas Munch et al13 reported that this SNP was associated with 4 times greater risk of peripheral drusen. Other SNPs have also been implicated. For example, Seddon et al14 found that peripheral subretinal drusenoid deposits and reticular pigment were associated with CFH rs1410996 in addition to CFH Y402H. These studies support that there are common genetic underpinnings and predisposing factors toward developing drusen and pigmentary changes in the macula and peripherally. Despite this, others have reported contradicting results,15 thus further research into this topic is necessary.
Fundus Autofluorescence
In addition to color imaging, fundus autofluorescence (FAF) has also been used to assess peripheral changes in AMD. Peripheral FAF abnormalities were found to be more common in neovascular AMD (86%) than non-neovascular AMD (73%) or controls (18%).5
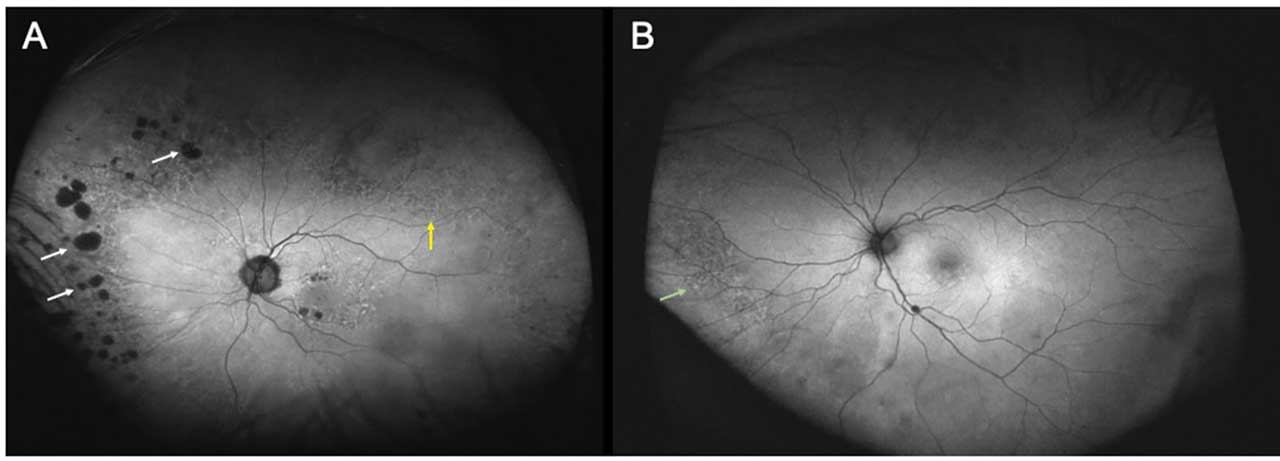
Fundus autofluorescence can highlight reticular pigmentary changes, reticular pseudodrusen, and cobblestone degeneration. Fundus autofluorescence patterns have also been reported in eyes with AMD. Tan et al showed that the most common peripheral FAF abnormality seen (46% of the cases) was granular FAF — small, discrete areas of bright, increased hyperautofluorescence (Figure 3A). This pattern was correlated clinically with evidence of peripheral drusen. The next most common pattern (34% of the cases) was mottled decreased FAF, which correlated clinically with RPE depigmentation, and is defined as generally decreased autofluorescence in an uneven/irregular pattern (Figure 3B). Nummular decreased FAF, or small to medium areas of discrete, uniformly decreased autofluorescence, was seen in 18% (Figure 3A).
Visual Function
The relationship between peripheral retinal changes and functional vision in AMD has also been an area of active investigation. Until recently, the majority of studies in AMD used visual acuity as a measure of disease severity and patient functioning. However, visual acuity tends to be unaffected until the later stages of the disease and thus is an imperfect metric, particularly for earlier disease and disease progression. Functional measures including dark adaptation, contrast sensitivity, and microperimetry have been correlated to macular changes in AMD.16-18 More recent research has suggested that peripheral alterations may also influence visual functioning in AMD. Lains et al19 demonstrated an association of peripheral alterations, such as midperipheral and far-peripheral reticular pigmentary changes, with delayed dark adaptation supporting that AMD changes with a functional impact are not localized only to the macula.
Challenges in Peripheral Retinal Assessment
Although UWF imaging has significantly improved peripheral assessment, it is not without its limitations. The quality of peripheral images can vary depending on factors including patient cooperation, media opacities, and image processing techniques. Additionally, images may be distorted peripherally. Addressing these technical challenges is essential for obtaining reliable and clinically relevant information from peripheral imaging.
Another challenge lies in the interpretation of peripheral retinal changes, as there is currently no universally accepted classification system for these lesions. As described above, there have been varied definitions of what the periphery entails, and there are various grids proposed but none adopted as the standard. Additionally, these grids, such as the OPERA Grid or the Boston Grid, each have their own advantages and disadvantages. Interobserver variability in identifying and grading these changes can also lead to discrepancies in clinical assessments.
Integrating peripheral retinal findings into the management of patients with AMD presents a unique set of challenges given that this is still a burgeoning area of investigation. Further work should focus on determining the clinical significance of peripheral changes, establish guidelines for monitoring, and decide if and how to incorporate these findings into management decisions.
Future Directions and Research
The field of peripheral retinal imaging continues to evolve, with promising developments on the horizon. Innovations in imaging modalities, such as improved UWF technology, hold the potential to enhance our ability to detect and monitor peripheral retinal changes in AMD. Ultrawidefield peripheral OCT scanning is a new technology that is already commercially available, and has been shown to adequately image peripheral pathology without significant artifact.6,20-22 Peripheral OCT can thus be used to further characterize the extramacular changes seen in AMD.
Another potential avenue for future research is in artificial intelligence and machine learning. These technologies can assist in automating the identification and quantification of peripheral retinal changes, reducing interobserver variability, and improving the efficiency of clinical assessments. Standardized protocols for grading and interpretation should also be established.
Longitudinal studies that track peripheral retinal changes over time are essential for understanding their role in the progression of AMD. Investigating how peripheral abnormalities correlate with macular disease development and whether they can serve as predictive markers for progression is also a critical area of ongoing research.
To better appreciate the clinical relevance of peripheral retinal changes in AMD, research is also needed to establish clear links between these changes and functional vision impairment. Studying the impact of peripheral alterations on critical visual functions, such as dark adaptation and contrast sensitivity, can provide valuable insights into their significance.
Conclusion
In recent years, UWF imaging has enabled a better understanding of peripheral changes in AMD and several grids and classification schemes have been proposed. Data support that peripheral retinal changes are more commonly seen in patients with AMD than age-matched controls, and that their presence may contribute to impaired visual function in these patients. Even though no standardized methods have been adopted, retina specialists should be aware of peripheral changes in patients with AMD and to evaluate the periphery as part of their comprehensive exams. Advances in imaging technology and ongoing research efforts are likely to further elucidate how peripheral retinal changes in AMD may affect clinical management. RP
Editor’s note: This article is discussed on the Retina Podcast at www.retinapodcast.com.
References
1. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. doi:10.1016/S2214-109X(13)70145-1
2. Ung C, Lains I, Miller JW, Kim IK. Current management of age-related macular degeneration. Adv Exp Med Biol. 2021;1256:295-314. doi:10.1007/978-3-030-66014-7_12
3. Forshaw TRJ, Minör ÅS, Subhi Y, Sørensen TL. Peripheral retinal lesions in eyes with age-related macular degeneration using ultra-widefield imaging: a systematic review with meta-analyses. Ophthalmol Retina. 2019;3(9):734-743. doi:10.1016/j.oret.2019.04.014
4. Klufas MA, Yannuzzi NA, Pang CE, et al. Feasibility and clinical utility of ultra-widefield indocyanine green angiography. Retina. 2015;35(3):508-520. doi:10.1097/IAE.0000000000000318
5. Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology. 2013;120(6):1271-1277. doi:10.1016/j.ophtha.2012.12.002
6. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3(10):843-849. doi:10.1016/j.oret.2019.05.007
7. Lengyel I, Csutak A, Florea D, et al. A population-based ultra-widefield digital image grading study for age-related macular degeneration-like lesions at the peripheral retina. Ophthalmology. 2015;122(7):1340-1347. doi:10.1016/j.ophtha.2015.03.005
8. Writing Committee for the OPTOS PEripheral RetinA (OPERA) study (Ancillary Study of Age-Related Eye Disease Study 2), Domalpally A, Clemons TE, et al. Peripheral retinal changes associated with age-related macular degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology. 2017;124(4):479-487. doi:10.1016/j.ophtha.2016.12.004
9. Oellers P, Laíns I, Mach S, et al. Novel grid combined with peripheral distortion correction for ultra-widefield image grading of age-related macular degeneration. Clin Ophthalmol. 2017;11:1967-1974. doi:10.2147/OPTH.S143246
10. Elwood KF, Richards PJ, Schildroth KR, Mititelu M. Peripheral exudative hemorrhagic chorioretinopathy (PEHCR): diagnostic and therapeutic challenges. Medicina (Kaunas). 2023;59(9):1507. doi:10.3390/medicina59091507
11. Choi EY, Kim HR, Jung J, Byeon SH, Kim SS, Kim M. Bilateral macular choroidal abnormalities with drusenoid deposits in patients with unilateral peripheral exudative hemorrhagic chorioretinopathy. Retina. 2023;43(1):120-129. doi:10.1097/IAE.0000000000003636
12. Shuler RK Jr, Schmidt S, Gallins P, et al. Peripheral reticular pigmentary change is associated with complement factor H polymorphism (Y402H) in age-related macular degeneration. Ophthalmology. 2008;115(3):520-524. doi:10.1016/j.ophtha.2007.06.021
13. Munch IC, Ek J, Kessel L, et al. Small, hard macular drusen and peripheral drusen: associations with AMD genotypes in the Inter99 Eye Study. Invest Ophthalmol Vis Sci. 2010;51(5):2317-2321. doi:10.1167/iovs.09-4482
14. Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci. 2009;50(2):586-591. doi:10.1167/iovs.08-2514
15. Ersoy L, Schick T, de Graft D, et al. Extramacular drusen are highly associated with age-related macular degeneration, but not with CFH and ARMS2 genotypes. Br J Ophthalmol. 2016;100(8):1047-1051. doi:10.1136/bjophthalmol-2015-306806
16. Neely D, Zarubina AV, Clark ME, et al. Association between visual function and subretinal drusenoid deposits in normal and early age-related macular degeneration eyes. Retina. 2017;37(7):1329-1336. doi:10.1097/IAE.0000000000001454
17. Tran BK, Herbort CP Jr. Discrepancy between visual acuity and microperimetry in AMD patients: visual acuity appears as an inadequate parameter to test macular function. Klin Monbl Augenheilkd. 2015;232(4):529-532. doi:10.1055/s-0035-1545779
18. Laíns I, Miller JB, Park DH, et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology. 2017;124(9):1340-1352. doi:10.1016/j.ophtha.2017.03.061
19. Laíns I, Park DH, Mukai R, et al. Peripheral changes associated with delayed dark adaptation in age-related macular degeneration. Am J Ophthalmol. 2018;190:113-124. doi:10.1016/j.ajo.2018.03.035
20. Ong J, Zarnegar A, Corradetti G, Singh SR, Chhablani J. Advances in optical coherence tomography imaging technology and techniques for choroidal and retinal disorders. J Clin Med. 2022;11(17):5139. doi:10.3390/jcm11175139
21. Stanga PE, Pastor-Idoate S, Reinstein U, et al. Navigated single-capture 3D and cross-sectional wide-field OCT of the mid and peripheral retina and vitreoretinal interface [published correction appears in Eur J Ophthalmol. 2022 Sep 13;11206721221126059]. Eur J Ophthalmol. 2022;32(3):1642-1651. doi:10.1177/11206721211026100
22. McNabb RP, Grewal DS, Mehta R, et al. Wide field of view swept-source optical coherence tomography for peripheral retinal disease. Br J Ophthalmol. 2016;100(10):1377-1382. doi:10.1136/bjophthalmol-2015-307480









