Neovascular age-related macular degeneration (nAMD) and diabetic retinopathy (DR), including diabetic macular edema (DME), are among the leading causes of vision loss.1 Standard of care for these individuals includes intravitreal anti-VEGF injections, which are effective in improving anatomic and visual outcomes.2,3 However, these frequent injections are a burden to patients and providers.4 Treatment paradigms like treat-and-extend have shown efficacy in expanding injection intervals.5 Also, recent innovations such as faricimab (Vabysmo; Genentech), which has dual targeting of Ang-2 and VEGF, have been shown to provide noninferior outcomes while extending injection intervals to every 16 weeks.6,7 High-dose aflibercept (Eylea HD; Regeneron) demonstrated a similar reduction in treatment burden.8,9 A greater degree of extension can also be achieved with the port delivery system (Susvimo; Genentech). However, port delivery is a surgical procedure with a relatively high risk of endophthalmitis, and it was voluntarily recalled by the company due to septum dislodgement.10 Current treatment modalities are still limited in several measures, including high treatment burden for most patients.
Mechanism of Action
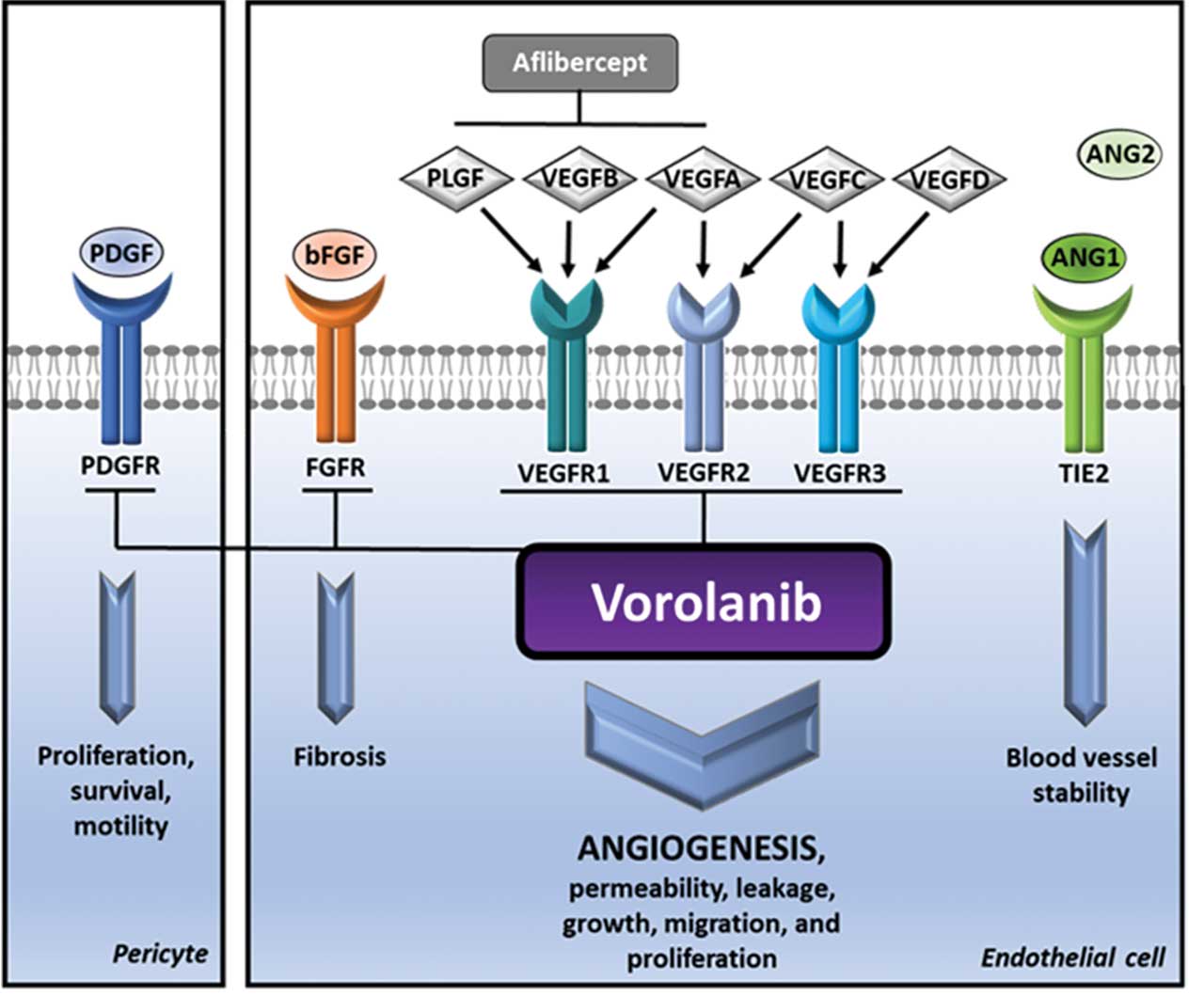
Anti-VEGF therapies target VEGF, the primary driver of nAMD and DR, and prevent its binding to the extracellular portion of the VEGF receptor (VEGFR). VEGFR is a receptor tyrosine kinase (RTK). Binding of VEGF to VEGFR causes RTK dimerization.11 This results in transphosphorylation of the tyrosine kinase domain in each RTK, initiating cellular pathways that drive vessel growth and permeability.12 Other RTKs involved in the pathogenesis of nAMD and DR include PDGFR, FGFR, and Tie2.13 Tyrosine kinase inhibitors (TKIs) are small molecules that diffuse into cells and act intracellularly to prevent tyrosine kinase phosphorylation and the resultant signaling cascade.12,13 Although both anti-VEGF and TKIs can suppress VEGFR activation, TKIs act on multiple tyrosine kinase receptors and at a different site on the receptor.12 Additionally, small molecules are rapidly cleared from the vitreous cavity, and as such, TKIs injected into the eye would not provide long-term suppression of neovascularization.14 This article will discuss sustained delivery TKIs currently in clinical trials and explore their potential as innovative treatment options for nAMD and DME (Figure 1).15
EYP-1901
EYP-1901 (EyePoint Pharmaceuticals) is an intravitreally injectable insert containing vorolanib.16 The delivery platform, Durasert E, is fully bioerodible and is fabricated using polymerized polyvinyl alcohol.16,17 Administration results in immediate vorolanib bioavailability through an initial burst of the drug followed by approximately 9 months of zero-order kinetic release. Following complete drug depletion, the matrix fully erodes.16 Vorolanib has been shown to inhibit multiple RTKs, such as VEGFR, PDGFR, and FGFR, while demonstrating minimal affinity for the Tie2 receptor.15,16
The DAVIO trial was a phase 1, single-injection, dose-escalation clinical trial of EYP-1901 in 17 previously treated nAMD patients. Patients were split into 4 cohorts of different dosages ranging from 440 µg to 3090 µg and were seen monthly for 12 months where they were assessed for rescue therapy need. Patients had 8.6 average annual injections at baseline and were able to decrease injection frequency by 77% at 6 months and by 73% at 12 months with 53% and 35% of patients remaining injection free at 6 and 12 months, respectively. Changes in best-corrected visual acuity (BCVA) and central subfield thickness (CST) were negligible, as were adverse events, with no reported ocular or drug-related serious adverse events (SAEs).18
DAVIO 2, a phase 2 multicenter, randomized, double-masked clinical trial in 160 patients with previously treated nAMD, compared 2 mg and 3 mg EYP-1901 to aflibercept with rescue treatment available.19 The primary outcome of change in vision at 28 and 32 weeks after EYP-1901 injection was met, with top-line data demonstrating noninferiority with the 2 mg and 3 mg groups gaining 1.0 and 0.9 EDTRS letters, respectively, and the aflibercept group improving by 1.3 EDTRS letters. There was an 89% and 85% reduction in treatment in the 2 mg and 3 mg groups, respectively, compared to the aflibercept group, and 65% and 64% of the treatment arms were able to remain rescue-free up to 6 months after EYP-1901 injection, respectively. The treatment arm provided anatomic control comparable to that of aflibercept and no drug-related SAEs were seen. The complete 12-month readout of DAVIO 2 is expected in the second half of 2024, and a global phase 3 trial in nAMD patients is planned to begin in the second half of 2024.15
The PAVIA trial, a current phase 2, randomized, double-masked study, will look at EYP-1901 in individuals with moderate to severe nonproliferative DR (NPDR) without center-involved diabetic macular edema and assess DRSS change at 36 weeks.20 This trial has enrolled 77 patients who have been randomly assigned to receive either 2 mg or 3 mg of EYP-1901 or a sham injection. Preliminary safety findings indicate no EYP-1901-related SAEs. Top-line data from the PAVIA study are expected in Q2 2024.7 VERONA is a single-masked, phase 2 trial of EYP-1901 in previously treated DME patients. Two EYP-1901 dosage arms (n=20) and an aflibercept control arm (n=5) will be followed for 24 weeks and evaluated for time to supplemental anti-VEGF injection (Figure 2).21

Axpaxli
Axpaxli (previously OTX-TKI; Ocular Therapeutix) is an intravitreal insert that delivers axitinib. This hydrogel is created using bioresorbable polyethylene glycol polymers that are crosslinked through trilysine linkers with axitinib enmeshed within the polymer matrix. After being injected through a 25-gauge needle, Axpaxli undergoes hydrolysis and fully bioresorbs at approximately 8 to 9 months.22Axitinib has been shown to selectively inhibit VEGF receptors and PDGF receptors and no Tie2 inhibition at clinically relevant tissue concentrations.23 In animal studies, the drug was delivered at zero-order kinetics and animals only exhibited minimal systemic axitinib exposure.24,25
A phase 1, dose-escalating trial evaluated Axpaxli in 23 patients with either untreated or previously treated nAMD.26,27 Patients were split into 4 cohorts and received treatment ranging from 200 µg to 600 µg of axitinib with rescue treatment available. Across all cohorts, 61% and 15% of patients did not require rescue treatment at 6 and 12 months, respectively. Axpaxli demonstrated a favorable safety profile with no reported SAEs.27
A phase 1b prospective, multicenter, randomized, double-masked clinical trial of a 0.6 mg Axpaxli implant was conducted in 21 patients with subfoveal neovascularization due to nAMD who were previously treated and had no excess retinal fluid.28 In this study, 16 patients received Axpaxli with aflibercept 4 weeks following, while the control group received a sham Axpaxli injection, followed by aflibercept 1 month later and then every 8 weeks thereafter with rescue treatment available. Patients had a mean of 8 anti-VEGF injections in the 12 months before study initiation. In the treatment arm, 73% and 33% of patients did not require rescue injection at 6 and 12 months after their last aflibercept injection, respectively. There was a sharp 27% increase in the need for rescue treatment toward the end of this trial, with 60% of patients remaining rescue free at 11 months, consistent with bioresorption of the implant at 8 to 9 months. Additionally, patients experienced an 89% reduction in treatment burden at 12 months. Among patients who required supplemental treatment, the median time to first injection was 44.6 weeks. By week 52, BCVA decreased by 1 EDTRS letter in the treatment arm and increased by 2 EDTRS letters in the control arm. There were no SAEs due to Axpaxli.23
HELIOS is a randomized, double-masked, phase 1b trial assessing the safety and efficacy of Axpaxli in 21 patients with moderate to severe NPDR without DME.29 Patients were randomized (2:1) to 600 µg of Axpaxli or sham injection. Patients will be followed for 12 months with top-line data expected in Q2 2024.30
SOL is a double-masked phase 3 clinical trial in treatment naïve nAMD patients using a reformulated Axpaxli that contains 450 μg more axitnib and employs a Form IV crystal polymorph form of axitinib. This trial will randomize 300 patients 1:1 to receive either Axpaxli or aflibercept after 2 monthly injections of aflibercept during the screening period. The primary endpoint will be the percentage of patients with <15 ETDRS letters of BCVA loss at week 36 with the plan to screen the first patient in Q1 2024.31
CLS-AX
CLS-AX (Clearside Biomedical) is a suprachoroidal (SC) injection of a proprietary durable axitinib suspension that is being tested in the treatment of nAMD. This suspension is administered through Clearside’s SCS Microinjector to deliver axitinib to the suprachoroidal space (SCS).32 A rabbit model study comparing SC delivery and intravitreal injections of an axitinib suspension found the SC delivery yielded 11-fold greater axitinib exposure to the posterior eye cup over 7 days. Additionally, in a pharmacokinetic rabbit study of bilateral SC administration, axitinib remained undetectable in the plasma and aqueous humor over the 3-month study.33
The OASIS and Extension Study was a 3-month and additional 3-month extension single injection, phase 1/2a clinical trial evaluating CLS-AX in previously treated nAMD patients (n=14) over a total of 6 months with rescue treatment available. The 3 treatment arms of the extension study were 0.1 mg (n=2), 0.5 mg (n=7), and 1.0 mg (n=5). Patients were anti-VEGF subresponders with an average of 10 annualized injections at baseline. When looking at all 14 patients and the 12 patients in the mid and high dosage arms, 57% and 67% of patients made it to 6 months without receiving rescue therapy, and 81% and 82% saw a reduction in treatment burden, respectively. No cases of vitreous floaters or SAEs were reported.32
ODYSSEY a randomized, double-masked phase 2b clinical trial, will assess CLS-AX in 60 patients with active nAMD judged to be anti-VEGF responders. Patients randomized (2:1) to the treatment arm will receive 1.0 mg of CLS-AX alongside their second of 3 loading doses of aflibercept while those in the aflibercept arm will only receive loading doses. Enrollment has been completed, and top-line results are anticipated for Q3 2024.34
D-4517.2
D-4517.2 (Ashvattha Therapeutics) is assigned to a new TKI class called dendranibs. This compound is administered subcutaneously or orally and is designed to treat nAMD and DME. This platform uses globular-shaped polyamidoamine dendrimers coated with hydroxyl groups.35 These hydroxyl dendrimers (HDs) are around 4 nm in diameter and bound to 7-8 proprietary sunitinib analog molecules.36 Animal models have demonstrated that systemically administered HDs cross the blood-retina barrier and selectively target areas of inflammation and neovascularization.37 Additionally, CNV mouse models showed HDs persist within lesions for >28 days and achieve near systemic clearance within 2 days.38 Further studies on CNV mouse models looked at CNV volume and found oral and subcutaneous D-4517.2 to significantly reduce neovascularization compared to vehicle and perform equivalently to or significantly better than intravitreal aflibercept.36,39
A dose-escalating, phase 1 clinical trial assessing single subcutaneous doses of D-4517.2 varying from 0.25 mg/kg to 1.0 mg/kg administered to healthy human participants (n=12) found them to be safe and well tolerated.38 An ongoing 2-stage phase 2 clinical trial is looking at a single subcutaneous D-4517.2 dose in 30 patients with 3 or 4 varying concentrations from 0.5 to 2 mg/kg or 0.25 to 2 mg/kg for DME and nAMD, respectively.40 Stage 1 results demonstrated safety and visual and anatomic outcomes suggestive of biological activity.41 Stage 2, or the phase 2 chronic dosing study, has been initiated with the aim to enroll at least 20 patients with nAMD or DME who will receive D-4517.2 every 2 or 4 weeks for up to 40 weeks, with preliminary data expected in the first half of 2024.42
PAN-90806
PAN-90806 (PanOptica) is a topical TKI eye drop.43 Phase 1/2 trials conducted in nAMD and PDR patients demonstrated biologic activity but caused punctate keratopathy. A new suspension was formulated and tested in a phase 1 and 2, dose-escalating, quadruple-masked, randomized clinical trial that looked at once-daily PAN-90806 use for 12 weeks in 51 treatment-naïve nAMD patients randomized (1:1:1) to receive 2, 6, or 10 mg/mL. There was a 79% reduction in injection burden, 51% of patients did not require rescue injection, and 88% of nonrescued patients experienced clinical improvement or disease stability. BCVA and CST remained stable throughout the study and no SAEs occurred.44 PanOptica entered into a license agreement with Zhaoke Ophthalmology Pharmaceutical Ltd. for PAN-90806 where its formulation is being optimized.45,46
Future Role
Anti-VEGF injections are the current mainstay of treatment in patients with nAMD and DR, including DME, especially in those who can reliably adhere to their scheduled injections.1,2 However, real-world studies indicate that patients receiving anti-VEGF therapy experience suboptimal visual outcomes compared to clinical trials, likely due to the high treatment burden and poor patient adherence.47,48 Clinical trials involving TKIs have reported promising outcomes, presenting a novel therapeutic approach that substantially diminishes the treatment burden for patients.
TKIs act at a different location than anti-VEGFs. They inhibit the intracellular domain of VEGFR, whereas anti-VEGF molecules bind to and prevent VEGF from acting on the extracellular domain of the VEGFR.12 To date, no studies of combined treatment with anti-VEGF and TKI have been conducted. However, treating neovascularization through both classes would achieve suppression of vessel growth and leakage through dual mechanism of action, which may provide benefit to certain patient populations such as those that are treatment resistant. Encouraging initial TKI clinical trial data point toward the effectiveness of these therapies in treating nAMD and DR. The advent of sustained-delivery TKIs represents a significant advancement, potentially reducing the frequency of treatments and thereby improving patient compliance and overall treatment effectiveness in real-world scenarios. RP
Hear discussion of this article on the Retina Podcast at https://www.retinalphysician.com/multimedia/podcasts/straight-from-the-cutters-mouth/.
References
- Centers for Disease Control and Prevention. Common eye disorders and diseases. Updated August 23, 2023. Accessed March 6, 2024. https://www.cdc.gov/visionhealth/basics/ced/index.html
- Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern. Ophthalmology. 2020;127(1):P66-P145. doi:1016/j.ophtha.2019.09.025
- Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern. Ophthalmology. 2020;127(1):P1-P65. doi:1016/j.ophtha.2019.09.024
- Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234-247. doi:10.1016/j.ophtha.2020.07.060
- Payne JF, Wykoff CC, Clark WL, et al. Randomized trial of treat and extend ranibizumab with and without navigated laser vs monthly dosing for diabetic macular edema: TREX-DME 2-year outcomes. Am J Ophthalmol. 2019;202:91-99. doi:10.1016/j.ajo.2019.02.005
- Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399(10326):741-755. doi:10.1016/S0140-6736(22)00018-6
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729-740. doi:10.1016/S0140-6736(22)00010-1
- Lanzetta P, Schulze A, Schmidt-Ott U, et al. Intravitreal aflibercept 8 mg injection in patients with neovascular age-related macular degeneration: 60-week and 96-week results from the phase 3 PULSAR trial. Presented at: the 23rd European Society of Retina Specialists Congress; October 5-8, 2023; Amsterdam, the Netherlands.
- Do DV. Aflibercept 8 mg for diabetic macular edema: 2-year results of the phase 2/3 PHOTON trial. Presented at: American Society of Retina Specialists annual meeting; July 28-August 1, 2023; Seattle, WA.
- Sharma A, Khanani AM, Parachuri N, Kumar N, Bandello F, Kuppermann BD. Port delivery system with ranibizumab (Susvimo) recall — what does it mean to the retina specialists. Int J Retina Vitreous. 2023;9(1):6. doi:10.1186/s40942-023-00446-z
- Ahmad A, Nawaz MI. Molecular mechanism of VEGF and its role in pathological angiogenesis. J Cell Biochem. 2022;123(12):1938-1965. doi:10.1002/jcb.30344.
- Esteban-Villarrubia J, Soto-Castillo JJ, Pozas J, et al. Tyrosine kinase receptors in oncology. Int J Mol Sci. 2020;21(22):8529. doi:10.3390/ijms21228529
- Vishwakarma S, Kaur I. Molecular mediators and regulators of retinal angiogenesis. Semin Ophthalmol. 2023;38(2):124-133. doi:10.1080/08820538.2022.2152706.
- Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;33(1):31-36. doi:10.1159/000055638
- Howard-Sparks M, Saim S, Farjo R, Paggiarino D, Duker JS, Lurker N. Neuroprotective effect of tyrosine kinase inhibitor vorolanib in a mouse model of retinal detachment. Presented at: Association for Research in Vision and Ophthalmology meeting; April 23-27, 2023; New Orleans, LA.
- Hershberger, V. The DAVIO2 trial: a phase 2, multicenter study of a single injection of EYP-1901 (vorolanib in the durasert E technology) vs aflibercept for previously treated wet age-related macular degeneration. Presented at: Hawaiian Eye and Retina; January 13-19, 2024; Wailea, HI.
- Khanani AM, Regillo CD, Wykoff CC, et al. Intravitreal sustained release tyrosine kinase inhibitors for the treatment of nAMD. Ocular Therapeutix supplement. Ophthalmology Management. 2023;27(1):S1-S12. https://digital.ophthalmologymanagement.com/articles/intravitreal-sustained-release-tyrosine-kinase-inhibitors-for-the-treatment-of-namd
- Abbey AM, Patel S, Barakat MR, et al. The DAVIO trial: a phase 1, open-label, dose-escalation study of a single injection of EYP-1901 (vorolanib in durasert platform) demonstrating reduced treatment burden in wet age-related macular degeneration. Presented at: Association for Research in Vision and Ophthalmology; April 23-27,2023; New Orleans, LA.
- Study of EYP-1901 in subjects with wet age-related macular degeneration (wAMD) (DAVIO2). ClinicalTrials.gov identifier: NCT05381948. Updated July 19, 2023. Accessed March 6, 2024.
- Study of EYP-1901 in patients with nonproliferative diabetic retinopathy (NPDR). ClinicalTrials.gov identifier: NCT05383209. Updated February 15, 2023. Accessed March 6, 2024.
- Duker J. JP Morgan Healthcare Conference presentation. January 10, 2024. Accessed March 6, 2024. https://investors.eyepointpharma.com/static-files/42a22e27-74bb-4d7c-a0f9-e3877cb5ec04
- Khanani AM, Couvillion SS, Eichenbaum DA, Steinle NC, Wykoff CC, Xavier S. 12-month update on randomized, controlled trial of OTX-TKI (axitinib intravitreal implant) for the treatment of wet AMD. Presented at: Clinical Trials at the Summit; June 10, 2023; Park City, UT.
- Avery RL. OTX-TKI, sustained-release axitinib hydrogel implant, for neovascular age-related macular degeneration. Presented at: the Retina Society Annual Meeting; October 13, 2023; New York, NY.
- Jarrett PK, Elhayek RF, Kahn E, Takach S, Metzinger JL, Goldstein MH. Efficacy and tolerability of OTX-TKI, a sustained hydrogel delivery system for a tyrosine kinase inhibitor, in a VEGF-induced retinal leakage model: 1 year results. Invest Ophthalmol Vis Sci. 2019;60(9):372.
- Kahn E, Patel C, Priem M, et al. A safety and pharmacokinetic study of a novel hydrogel-based axitinib intravitreal implant (OTX-TKI) in non-human primates. Invest Ophthalmol Vis Sci. 2022;63(7):297-F0100.
- CLN-0046: treatment of AMD subjects with OTX-TKI. ClinicalTrials.gov identifier: NCT03630315. Updated August 3, 2022. Accessed March 6, 2024.
- Moshfeghi AA. Australia-based Phase 1 Trial of a novel, hydrogel-based, intravitreal Axitinib implant for the treatment of neovascular age-related macular degeneration. Presented at: American Academy of Ophthalmology; September 30-October 3, 2022; Chicago, IL.
- Study evaluating the treatment of OTX-TKI for subjects with neovascular age-related macular degeneration. ClinicalTrials.gov identifier: NCT04989699. Updated September 6, 2022. Accessed March 6, 2024.
- Study to evaluate the safety, tolerability, and efficacy of OTX-TKI in subjects with moderately severe to severe non-proliferative diabetic retinopathy. ClinicalTrials.gov identifier: NCT05695417. Updated December 8, 2023. Accessed March 6, 2024.
- Ocular Therapeutix, Inc. Corporate presentation. January 2024. Accessed March 6, 2024. https://investors.ocutx.com/static-files/c8a4064b-8271-49db-ab5e-03a075777552
- Ocular Therapeutix, Inc. Ocular Therapeutix receives FDA agreement under special protocol assessment (SPA) for its first pivotal clinical trial of OTX-TKI in wet AMD. Press release. November 1, 2023. Accessed March 6, 2024. https://investors.ocutx.com/news-releases/news-release-details/ocular-therapeutixtm-receives-fda-agreement-under-special
- Clearside Biomedical, Inc. Corporate presentation. May 2023. Accessed March 6, 2024. https://ir.clearsidebio.com/static-files/3c2f7b9f-cdd2-4a79-a22e-4d15471d57d2
- Kansara VS, Muya LW, Ciulla TA. Evaluation of long-lasting potential of suprachoroidal axitinib suspension via ocular and systemic disposition in rabbits. Transl Vis Sci Technol. 2021;10(7):19. doi:10.1167/tvst.10.7.19
- Clearside Biomedical, Inc. Corporate presentation. January 2024. Accessed March 6, 2024. https://ir.clearsidebio.com/static-files/d534179e-8bab-4a19-a899-555032a0ea1b
- Ocular Therapeutix, Inc. Targeted nanomedicine: Ashvattha Therapeutics’ path to developing hydroxyl dendrimers. Accessed March 6, 2024. https://avttx.com/wp-content/uploads/WhitePaper_FINAL_Apr2023.pdf
- Cleland JL, Sharma R, La Rosa Appiani S, et al. Single subcutaneous (or oral) dose of anti-angiogenesis drug safely suppresses choroidal neovascularization comparable to intravitreal aflibercept. Presented at: Association for Research in Vision and Ophthalmology meeting; May 2-6, 2021; San Francisco, CA. https://avttx.com/wp-content/uploads/Ashvattha-ARVO-2021-Poster_Final.pdf
- Kambhampati SP, Bhutto IA, Wu T, et al. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J Control Release. 2021;335:527-540. doi: 10.1016/j.jconrel.2021.05.035.
- Cleland JL, Sharma, R, Appiani La Rosa S, Moore J, Rogers B. Safety and tolerability of a single subcutaneous dose of anti-angiogensis drug to treat age-related macular degeneration (wet AMD) and diabetic retinal edema. Presented at: Association for Research in Vision and Ophthalmology meeting; May 1-4, 2022; Denver, CO. https://avttx.com/wp-content/uploads/Ashvattha-ARVO-2022-Poster_Final.pdf
- Le Moan N, Culp DW, Marjoram L, Dwadasi V, Cleland J. Oral formulation development of the anti-angiogenesis drug D-4517.2 to treat age-related macular degeneration (wet AMD) and diabetic macular edema (DME). Invest Ophthalmol Vis Sci. 2023;64(8):1299.
- A study to evaluate the safety, tolerability and Ppharmacokinetics of D-4517.2 after subcutaneous administration in subjects with neovascular (wet) age-related macular degeneration (AMD) or subjects with diabetic macular edema (DME) (Tejas). ClinicalTrials.gov identifier: NCT05387837. Updated February 13, 2023. Accessed March 6, 2043.
- Ashvattha Therapeutics. D-4517.2 ophthalmology pipeline represents a paradigm shift in treatment. Accessed March 6, 2024. https://avttx.com/pipeline/ophthalmology/
- Ashvattha Therapeutics. Ashvattha Therapeutics announces first patient dosed via subcutaneous administration of anti-angiogenic therapeutic D-4517.2 for wet AMD and DME in phase 2 chronic dosing study. News release. November 1, 2023. Accessed March 6, 2024. https://avttx.com/ashvattha-therapeutics-announces-first-patient-dosed-via-subcutaneous-administration-of-anti-angiogenic-therapeutic-d-4517-2-for-wet-amd-and-dme-in-phase-2-chronic-dosing-study/
- Chaney P. PAN-90806: once-daily topical anti-VEGF eye drop for wet AMD and other neovascular eye disease. Presented at: Ophthalmology Innovation Summit; October 10, 2019; San Francisco, CA.
- Study of PAN-90806 eye drops, suspension for neovascular AMD. ClinicalTrials.gov identifier: NCT03479372. Updated July 9, 2019. Accessed March 6, 2024.
- Zhaoke Hong Kong and PanOptica entered into license agreement for PAN-90806. News release. December 1, 2020. Accessed March 6, 2024. https://www.zkoph.com/news?lang=en&sid=38
- Zhaoke Ophthalmology Announces 2022 Annual Results. News release. March 27, 2023. Accessed March 6, 2024. https://www.zkoph.com/news?lang=en&sid=78
- Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020; 4(1):19-30. doi:10.1016/j.oret.2019.05.017.
- Ghanchi F, Bourne R, Downes SM, et al. An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis, and glaucoma. Eye (Lond). 2022;36(6):1154-1167. doi:10.1038/s41433-021-01766-w.










