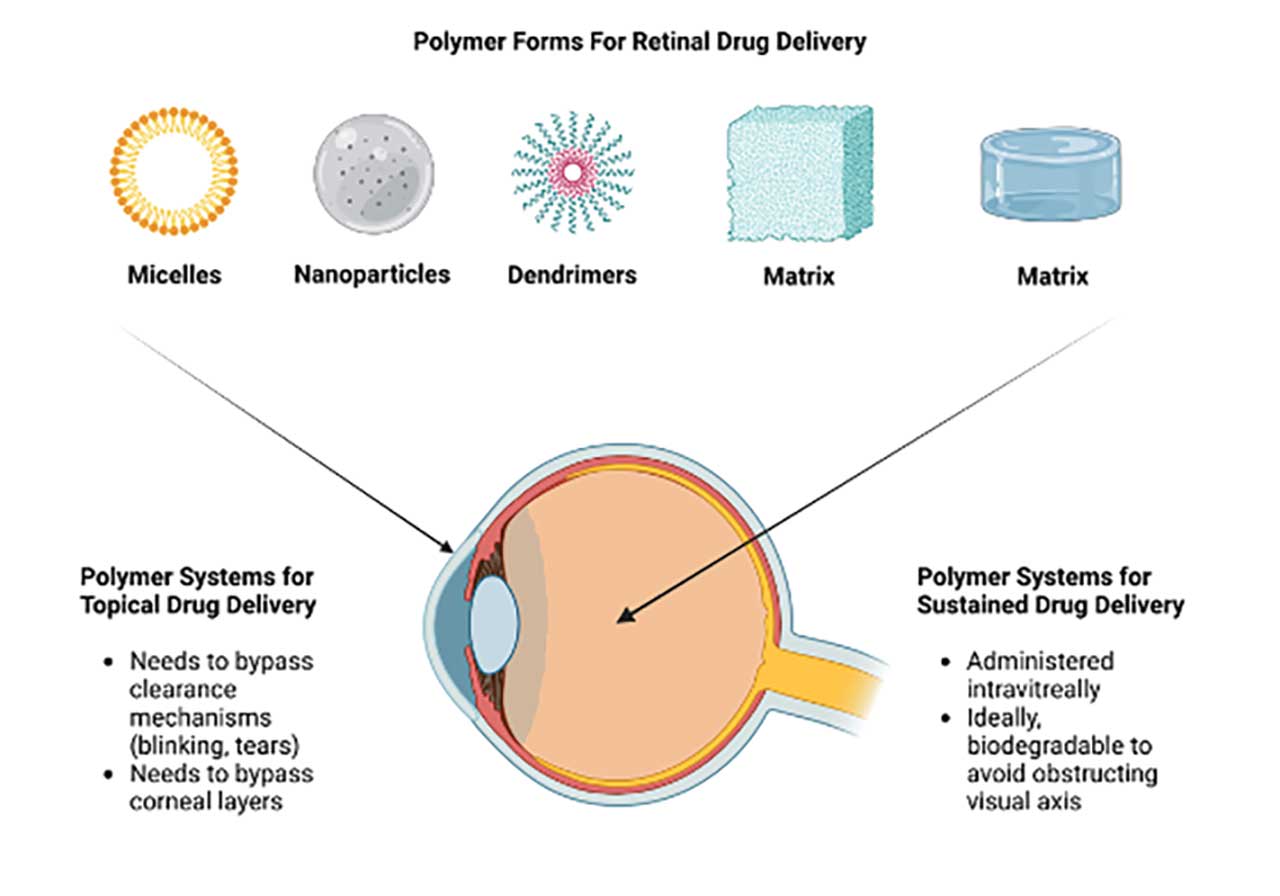
Polymers are large macromolecules made of repeating subunits known as monomers. These can be synthetic or natural. Due to their unique physicochemical properties, they have been harnessed for biomedical applications for decades. Common biomedical applications in the retinal space include vitreous substitutes, drug delivery platforms, and surgical adjuncts such as scleral buckles. Polymers can also come in various forms including nanoparticles, micelles, dendrimers, and hydrogels (Figure 1).
Despite years of innovation since intravitreal bevacizumab was first tested for neovascular age-related macular degeneration (nAMD),1 intravitreal administration of drugs remain the mainstay of treatment in retinal neovascular diseases like nAMD and diabetic macular edema (DME). These drugs include anti–vascular endothelial growth factor (anti-VEGF) and glucocorticoid compounds. Polymeric technologies have the potential to revolutionize this treatment approach. Many companies have leveraged the ever-expanding pipeline of academic biomaterial research to develop new therapies that can (1) reduce intravitreal injection frequency through sustained drug release platforms or (2) circumvent the need for injections through topical drug-delivery platforms. This article highlights the pipeline of retinal drugs that utilize polymeric technologies for these purposes (Table 1).
Table 1: Polymeric Technologies in Development for Sustained Retinal Drug Delivery
|
Therapeutic; Company |
Polymer |
Drug |
Administration |
Recent Clinical Trials |
Ongoing Retinal Programs |
|
OTX-TKI; Ocular Therapeutix |
Polyethylene glycol hydrogel |
Axitinib, tyrosine kinase inhibitor |
Intravitreal |
Phase 1 for nAMD (NCT04989699 and NCT03630315) |
Phase 3 for nAMD (NCT06223958) Phase 1 for NPDR (NCT05695417) |
|
KSI-301 |
Antibody-biopolymer conjugate IgG1 anti-VEGF A Phosphorylcholine biopolymer |
Intravitreal |
Phase 3 for DME (GLEAM, NCT04611152; GLIMMER, NCT04603937) Phase 3 for NPDR (GLOW, NCT05066230) Phase 3 for nAMD (DAYLIGHT, NCT04964089) Phase 3 for macular edema due to retinal vein occlusion (BEACON, NCT04592419) |
|
|
|
EYP-1901; EyePoint Pharmaceuticals |
Durasert, polyimide matrix |
Vorolanib, tyrosine kinase inhibitor |
Intravitreal |
Phase 2 for nAMD (DAVIO2, NCT05381948) |
Phase 2 trial for DMO (VERONA, NCT06099184) Phase 2 trial for NPDR (PAVIA, NCT05383209) |
|
Brimo Drug Delivery System; Allergan/AbbVie |
Poly(D,L-lactic-co-glycolic acid)/(D,L-lactic acid) matrix |
Brimonidine, alpha-2- |
Intravitreal |
Phase 2b for geographic atrophy (BEACON, NCT02087085) |
Planned for phase 3 trials using 200 μg and 400 μg dosages (IMAGINE and ENVISION) |
|
PER-001; Perfuse Therapeutics |
Polylactic-co-glycolic acid matrix |
Endonentan, endothelin A receptor antagonist |
Intravitreal |
NA |
Phase 1/2a study for advanced open-angle glaucoma (NCT05822245) Phase 2a trial for DR (NCT06003751) |
|
OCS-01; Oculis |
Cyclodextrin nanoparticle |
Dexamethasone |
Topical |
NA |
Phase 2/3 trial for diabetic macular edema (DIAMOND, NCT05066997) |
Sustained Drug Release in the Posterior Segment
For the treatment of neovascular retinal diseases, current FDA-approved intravitreal therapeutics are administered monthly to every 3 months. Reducing the frequency of these injections can improve treatment compliance and reduce the incidence of complications such as vitreous hemorrhage, retinal detachment, and endophthalmitis.
Polymeric technologies have been harnessed for such applications. For instance, Ozurdex (Allergan/AbbVie) is a biodegradable, dexamethasone intravitreal implant that was approved in 2009 for macular edema from retinal vein occlusion (RVO). This has since expanded to posterior-segment uveitis and DME. The implant is made of polylactic-co-glycolic acid (PLGA), which hydrolyses into lactic acid and glycolic acid when exposed to moisture in the vitreous cavity. The hydrolysis also releases the stored dexamethasone. The implant can sustain drug delivery up to 6 months, with a peak effectiveness typically between 60 and 90 days.
In a bid to further prolong glucocorticoid release, Iluvien (Alimera Sciences) was designed as a nonbiodegradable fluocinolone acetonide intravitreal implant with a longer release profile. First approved for the treatment of DME in 2014, the implant is a tiny cylindrical tube approximately 3.5 mm in length and 0.37 mm in diameter. The tube is a polyvinyl alcohol matrix that contains the drug. Upon insertion into the posterior chamber through the pars plana, the matrix becomes hydrated and the drug slowly diffuses out of the implant. The implant is engineered to release the drug at 0.2ug/day.2 In the postapproval phase 4 monitoring study, PALADIN, 25% of patients did not require any additional treatments up to 3 years in duration.3
Upcoming Polymeric Sustained Drug-release Platforms
Despite the above-mentioned therapeutics, there remains an unmet clinical need for a safe and effective sustained drug-release platform. Intraocular glucocorticoids can lead to raised intraocular pressure (IOP), which requires monitoring via clinic visits. Hence, research on polymeric approaches have led to the next generation of platforms that deliver novel drug, such as tyrosine kinase inhibitors (TKIs). The following highlights the upcoming pipeline of polymeric sustained drug-release platforms.
OTX-TKI
OTX-TKI (Ocular Therapeutix) is an investigational, bioresorbable hydrogel implant made of polyethylene glycol (PEG) that contains axitinib, a TKI. It is designed for intravitreal administration and is currently being developed for both diabetic retinopathy (DR) and neovascular AMD. Due to its biocompatibility, PEG is one of the most common polymers used in the development of FDA-approved therapeutics and devices. The implant has a high solubility due to the hydrolysable linkages in the hydrogel structure that break down over time to a soluble polymer that is cleared from the vitreous. In the process, the drug is released from the hydrogel.
OTX-TKI is currently in the late stage of clinical development. In separate nAMD phase I trials conducted in the United States and Australia, no serious adverse events, including retinal detachment, raised IOP, or endophthalmitis were observed in both treatment-naïve and treated patients.4,5 A pivotal, multicenter, randomized trial is currently ongoing for nAMD, comparing aflibercept with OTX-TKI.6 At the same time, the interim results from the HELIOS pilot trial involving moderately severe to severe nonproliferative DR (NPDR) are scheduled to be released soon.7
KSI-301
KSI-301 (Kodiak Biosciences) is an investigational anti-VEGF antibody-biopolymer conjugate that is currently undergoing development for nAMD, DME and macular edema due to RVO. The therapeutic consists of a IgG1 antibody that is conjugated to a phosphorylcholine polymer. Phosphorylcholine is organically found in the cell membranes. Due to the polymer’s hydrophilic nature, the binding sites of the antibody are protected from nonspecific interactions, thereby prolonging the half-life and reducing treatment frequency.
In the previous GLEAM and GLIMMER phase 3 studies, KSI-301 failed to meet the best-corrected visual acuity (BCVA) primary endpoint when compared to aflibercept in treatment-naïve patients with DME.8-10 KSI-301 was also trialed in 3 other phase 3 studies, namely the GLOW study for NPDR, DAYLIGHT study for nAMD, and BEACON study for macular edema due to RVO.11-13 In these studies, KSI-301 met the primary endpoints. However, it should be noted that the DAYLIGHT study compared a 4-week frequency of KSI-301 with an 8-week frequency of aflibercept. Hence, treatment burden may still be an issue for nAMD patients. Interestingly, in GLOW, where KSI-301 was compared with sham injections for the treatment of moderate and severe NDPR, the KSI-301 arm had a significantly larger proportion of eyes improving on the DR severity scale. While the development of KSI-301 was initially halted after the release of the GLEAM and GLIMMER study results, the company is planning to proceed with another pivotal trial after the positive results of BEACON and GLOW.
EYP-1901
EYP-1901 (EyePoint Pharmaceuticals) is an intravitreal implant that is made of a bioerodible version of Durasert, the polyimide technology that forms the basis of Iluvien. The implant releases vorolanib, a selective and potent pan-VEGF inhibitor. The therapeutic is being designed as a twice-yearly intravitreal therapeutic and is currently undergoing clinical trials for nAMD, NPDR, and DME. In preclinical studies, EYP-1901 could sustain release of voralanib over an 8-month period in rabbit models.
The therapeutic program for nAMD is the most advanced in development. In the most recent top-line results announcement from the phase 2 DAVIO2 trial, a single dose of EYP-1901 demonstrated noninferiority compared to a 8-weekly aflibercept regimen in terms of mean BCVA over a period of 32 weeks.14 No serious adverse events were reported. EYP-1901 is also currently being studied in 2 other phase 2 trials, VERONA for DME and PAVIA for NPDR.15,16 The results from PAVIA are expected to be released in the middle of 2024 and the company is currently planning for a phase 3 nAMD trial based on the positive results from DAVIO2.
Brimo Drug Delivery System
Brimo Drug Delivery System (Brimo DDS; Allergan/AbbVie) is a biodegradable polymer implant containing brimonidine that is currently being developed for geographic atrophy (GA). While brimonidine, an alpha-2 adrenergic agonist, is typically used in glaucoma, it was also noted to protect photoreceptor and retinal pigment epithelial cells against oxidative and phototoxic damage. Hence, it is currently being developed for GA where there is a significant need for novel therapeutics. Since its initial development, the implant has undergone changes to improve the amount of drug loading and release kinetics. First, the initial drug, brimonidine tartrate, was substituted with brimonidine free base. This allows a higher amount of drug to be loaded on the DDS. Secondly, the initial implant matrix was made of poly-(D,L-lactide) polymer. This was subsequently converted to a poly(D,L-lactic-co-glycolic acid)/(D,L-lactic acid) blend that has faster drug release.
In the latest phase 2b BEACON study, GA patients were randomized to receive either Brimo DDS or sham injections.17 The primary endpoint of the study was to compare the GA lesion area change from baseline at 24 months between the 2 arms. Despite not meeting the primary endpoint, it was observed that there was a significant decrease in GA growth at 24 and 30 months.18 Currently Brimo DDS is planned to undergo another 2 phase 3 trials exploring 200 μg and 400 μg dosages (IMAGINE and ENVISION).
PER-001
PER-001 (Perfuse Therapeutics) is a biodegradable intravitreal implant made of a PLGA polymer matrix. The implant is loaded with endonentan, a highly selective and potent endothelin A receptor antagonist. Endothelin is a potent vasoconstrictor, and blocking such signalling can potentially ameliorate the downstream effects in the pathogenesis of ischemia-driven diseases. PER-001 is currently in clinical development for an IOP-independent therapeutic approach to glaucoma and DR.19
Data from the phase 1/2a study for advanced open-angle glaucoma showed that PER-001 was evaluated to be safe and well tolerated, thereby allowing the program to advance to the phase 2a portion of the study.20 A phase 2a trial for DR was subsequently announced.21 The study will be comparing the safety and tolerability of 2 dosages of PER-001 compared to sham injections in patients with DR.
Topical Delivery of Drugs to the Posterior Segment
Topical delivery of drugs to the posterior segment can circumvent the need for intravitreal drug administration. Patients can then continue treatment in the comfort of their home. However, for polymeric technologies to achieve this, various anatomic and physiologic hurdles will have to be overcome. First, the clearance mechanism of the eyes, including blinking and tearing, will have to be addressed. Second, the polymer platform will need to penetrate or bypass the various corneal layers to reach the posterior segment of the eye. Finally, the polymer platform should be biocompatible with tissues and cells found on the ocular surface of the eye. Polymeric technologies can address these issues by increasing the residence time of therapeutics on the corneal surface or allow penetration into the various corneal layers.
Upcoming Topical Drug Delivery Platforms
OCS-01 (Oculis) is a topical platform that uses the Optireach technology to improve the residence time and bioavailability of dexamethasone for the noninvasive treatment of retinal diseases. The delivery platform is a cyclodextrin nanoparticle. Cyclodextrins are polysaccharides that are commonly used to enhance the solubility of poorly soluble drugs. They were found to be able to adhere to the corneal surface and enhance penetration. At the same time, cyclodextrins are biocompatible with the corneal surface.22
OCS-01 is currently being evaluated in a phase 2/3 trial, DIAMOND, as a topical treatment for DME.23 The trial is a 2-stage trial with the first stage being 12 weeks before primary endpoint was evaluated. The second stage of the trial has the same primary endpoint but is evaluated at 1 year in duration. In the first stage of the trial, it was observed that there was a significant difference between OCS-01–treated eyes and vehicle-treated in terms of the mean change in BCVA from baseline to both the 6-week and 12-week timepoints. Concurrently, there was also a significant decrease in central subfield thickness across 12 weeks as compared to baseline in the OCS-01 treatment arm. However, due to the glucocorticoid nature of the delivered drug, 22% of the study cohort developed raised IOP. Stage 2 of the trial is currently ongoing.
Future Directions and Conclusions
The unique properties of polymeric technologies have revolutionized many aspects of clinical medicine. This is especially so in the field of drug delivery for therapeutic indications such as cardiovascular disease, radiology, and oncology. The pipeline of polymeric technologies being harnessed to solve the challenges associated with intravitreal drug administration is robust. With multiple ongoing phase 3 trials, retinal physicians will likely have an expanded repertoire of treatments to choose from as some of these novel polymer-based therapeutics obtain approval from the FDA. RP
References
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363-372.e5. doi:10.1016/j.ophtha.2005.11.019
- Massa H, Nagar AM, Vergados A, Dadoukis P, Patra S, Panos GD. Intravitreal fluocinolone acetonide implant (ILUVIEN) for diabetic macular oedema: a literature review [published correction appears in J Int Med Res. 2019 Feb;47(2):1099]. J Int Med Res. 2019;47(1):31-43. doi:10.1177/0300060518816884
- Singer MA, Sheth V, Mansour SE, Coughlin B, Gonzalez VH. Three-year safety and efficacy of the 0.19-mg fluocinolone acetonide intravitreal implant for diabetic macular edema: the PALADIN study. Ophthalmology. 2022;129(6):605-613. doi:10.1016/j.ophtha.2022.01.015
- Study evaluating the treatment of OTX-TKI for subjects with neovascular age-related macular degeneration. Clinicaltrials.gov identifier: NCT04989699. Updated January 26, 2024. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT04989699
- CLN-0046: treatment of AMD subjects with OTX-TKI. Clinicaltrials.gov identifier: NCT03630315. Updated August 3, 2022. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT03630315
- Study to evaluate the efficacy and safety of intravitreal OTX-TKI (Ocular Therapeutix) (axitinib implant) in subjects with neovascular age-related macular degeneration. Clinicaltrials.gov identifier: NCT06223958. Updated February 13, 2024. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT06223958
- Study to evaluate the safety, tolerability, and efficacy of OTX-TKI in subjects with moderately severe to severe non-proliferative diabetic retinopathy. Clinicaltrials.gov identifier: NCT05695417. Updated December 8, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT05695417
- A trial to evaluate the efficacy, durability, and safety of KSI-301 compared to aflibercept in participants with diabetic macular edema (DME) (GLEAM). Clinicaltrials.gov identifier: NCT04611152. Updated October 2, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT04611152
- A study to evaluate the efficacy, durability, and safety of KSI-301 compared to aflibercept in participants with diabetic macular edema (DME) (GLIMMER). Clinicaltrials.gov identifier: NCT04603937. Updated October 2, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT04603937
- Kodiak Sciences Inc. Kodiak Sciences announces topline results from its phase 3 studies of tarcocimab tedromer in neovascular age-related macular degeneration and diabetic macular edema and provides update on tarcocimab development program. News release. July 4, 2023. Accessed February 23, 2024. https://ir.kodiak.com/news-releases/news-release-details/kodiak-sciences-announces-topline-results-its-phase-3-studies
- A study to evaluate the efficacy and safety of intravitreal KSI-301 in participants with moderately severe to severe non-proliferative diabetic retinopathy (NPDR) (GLOW). Clinicaltrials.gov identifier: NCT05066230. Updated November 13, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT05066230
- A study to evaluate the efficacy and safety of intravitreal KSI-301 compared with intravitreal aflibercept in participants with neovascular (wet) age-related macular degeneration (wAMD) (DAYLIGHT). Clinicaltrials.gov identifier: NCT04964089. Updated May 30, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT04964089
- A study to evaluate the efficacy, durability, and safety of KSI-301 compared to aflibercept in patients with macular edema due to retinal vein occlusion (RVO) (BEACON). Clinicaltrials.gov identifier: NCT04592419. Updated February 9, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT04592419
- Study of EYP-1901 in subjects with wet age-related macular degeneration (wAMD) (DAVIO2). Clinicaltrials.gov identifier: NCT05381948. Updated February 22, 2024. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT05381948
- Study of EYP-1901 in patients with diabetic macular edema (DME) (VERONA). Clinicaltrials.gov identifier: NCT06099184. Updated December 5, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT06099184
- Study of EYP-1901 in patients with non-proliferative diabetic retinopathy (NPDR). Clinicaltrials.gov identifier: NCT05383209. Updated February 12, 2024. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT05383209
- Freeman WR, Bandello F, Souied E, et al. Randomized phase 2b study of brimonidine drug delivery system generation 2 for geographic atrophy in age-related macular degeneration. Ophthalmol Retina. 2023;7(7):573-585. doi:10.1016/j.oret.2023.03.001
- Lin CW, Glendenning AD, Gurkan S. Pharmaceutical compositions and intravitreal drug delivery systems for the treatment of ocular diseases. US Patent. April 6, 2023. Accessed February 23, 2024. https://patentimages.storage.googleapis.com/4f/d8/8b/21bd9b0f33e5de/US20230105507A1.pdf.
- A study of PER-001 in participants with open-angle glaucoma. Clinicaltrials.gov identifier: NCT05822245. Updated August 9, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT05822245
- Perfuse Therapeutics. Perfuse Therapeutics announces initiation of enrollment to ph2a portion of the phase 1/2a clinical trial of PER-001 intravitreal implant in patients with glaucoma. News release. July 20, 2023. Accessed February 26, 2024. https://perfusetherapeutics.com/perfuse-therapeutics-announces-initiation-of-enrollment-to-ph2a-portion-of-the-phase-1-2a-clinical-trial-of-per-001-intravitreal-implant-in-patients-with-glaucoma/
- A study of PER-001 in participants with diabetic retinopathy. Clinicaltrials.gov identifier: NCT06003751. Updated November 15, 2023. Accessed February 26, 2024. https://clinicaltrials.gov/study/NCT06003751
- Liu CH, Lee GW, Wu WC, Wang CC. Encapsulating curcumin in ethylene diamine-β-cyclodextrin nanoparticle improves topical cornea delivery. Colloids Surf B Biointerfaces. 2020;186:110726. doi:10.1016/j.colsurfb.2019.110726
- Multicenter study on the efficacy and safety of OCS-01 in subjects with diabetic macular edema. Clinicaltrials.gov identifier: NCT05066997. Updated January 17, 2023. Accessed February 23, 2024. https://clinicaltrials.gov/study/NCT05066997










