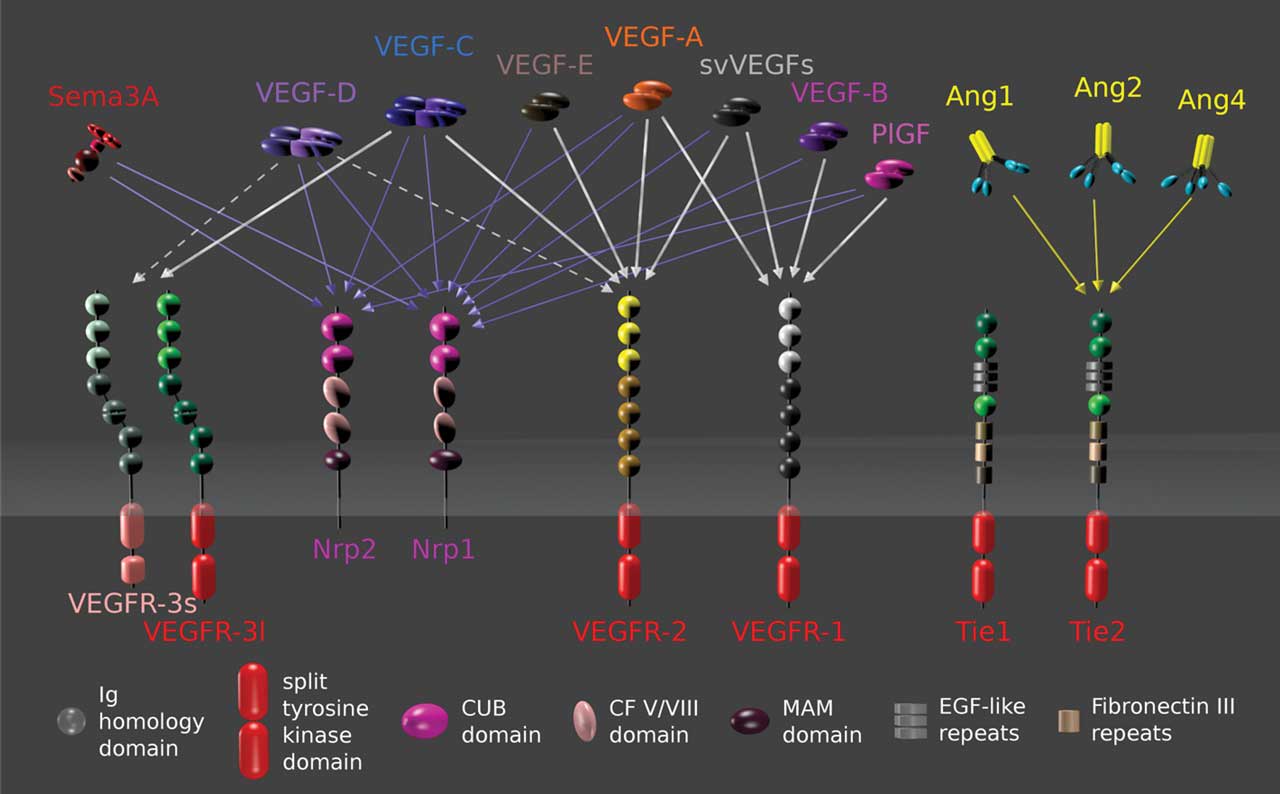
Over the past decade, intravitreal anti‒vascular endothelial growth factor (VEGF) injections have become the standard of care for treating neovascular age-related macular degeneration (nAMD).1–5 Real-world data show that patients who receive more frequent or continuous fixed-interval dosing can achieve favorable long-term vision preservation,6,7 while those who receive reduced injection frequency compared to clinical trials experience worse outcomes.8,9 Monthly or bimonthly anti-VEGF use, however, poses significant time and cost burden due to the disease’s chronic nature and limited medication half-life. Faricimab (Vabysmo; Genentech), a novel bispecific monoclonal antibody with inhibition of both VEGF-A and angiopoetin-2 (Ang-2) (Figure 1), was recently approved for nAMD and has demonstrated encouraging durability benefit with up to 16-week dosing intervals in clinical trials and initial real-world studies.10,11 Further potential solutions to address the need for long-acting and more durable therapies include high-concentration anti-VEGF drugs, sustained-release devices, intravitreal reservoirs, suprachoroidal delivery, and gene therapy biofactories.
Intravitreal High-Molar-Concentration Therapies
Brolucizumab (Beovu; Novartis Pharmaceuticals), a humanized small single-chain variable antibody fragment with inhibition of all VEGF-A isoforms at a low molecular weight of 26 kDa (Figure 2), was FDA approved for nAMD in October 2019. In the phase 3 HAWK and HARRIER trials, brolucizumab demonstrated both greater fluid resolution and the potential to maintain nAMD patients on an extended 3-month dosing schedule.2 Real-world studies have supported its efficacy and capacity to extend treatment intervals after switching from other anti-VEGF agents.12 However, commercial adoption has been tempered by rare incidents of significant vision loss secondary to intraocular inflammation.13
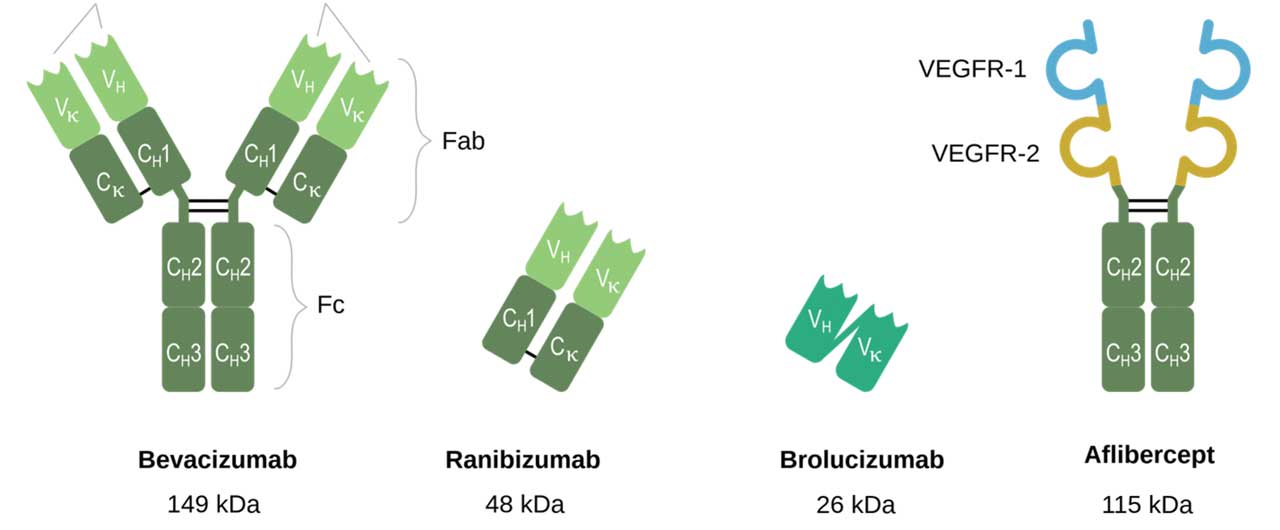
Aflibercept ( Eylea; Regeneron Pharmaceuticals ), a recombinant humanized fusion protein that blocks VEGF-A, VEGF-B, and placental growth factor (PlGF), was approved for nAMD in 2011 as a 2 mg/0.05 mL intravitreal injection administered up to every 8 weeks. Aflibercept 8 mg/0.07 mL (Eylea HD) gained approval for the treatment of nAMD in August 2023. The phase 3 PULSAR trial demonstrated noninferiority and clinically equivalent vision gains with 12-week and 16-week dosing regimens of Eylea HD compared to bimonthly aflibercept 2 mg.14
Vitreous Implants and Polymer-based Intravitreal Sustained Release
Vitreous implants with polymer-based sustained-release platforms may offer a solution for reducing the treatment burden in nAMD and other retinal disorders (Figure 3). Some intravitreal therapeutic implants involve a “tablet” surrounded by a polymer to control drug release into the vitreous, and other therapies involve drug enmeshed within a polymer to control drug release. Successful examples of nonerodible polymer-based delivery systems include a ganciclovir 4.5 mg implant (Vitrasert; Bausch + Lomb), a fluocinolone acetonide 0.59 mg implant (Retisert; Bausch + Lomb), a fluocinolone acetonide 0.19 mg implant (Iluvien; Alimera Sciences), and a fluocinolone acetonide 0.18 mg implant (Yutiq; Alimera Sciences).

The dexamethasone 0.7 mg implant (Ozurdex; Allergan/AbbVie) is an example of an erodible implant. Ozurdex has been investigated as an adjunct therapy to ranibizumab in nAMD; however, it has not demonstrated reproducible visual or anatomic benefits relative to anti-VEGF monotherapy.15,16
EYP-1901 (Eye-Point Pharmaceuticals) uses the bioerodible Durasert platform for sustained-delivery intravitreal injection of vorolanib, a tyrosine kinase inhibitor (TKI) with binding affinity to VEGF-A, VEGF-C, and VEGF-D. The phase 1 DAVIO study demonstrated favorable safety profiles for multiple doses of EYP-1901. Top-line interim results from the ongoing phase 2 DAVIO 2 trial revealed 2 mg and 3 mg doses of EYP-1901 achieved all primary and secondary endpoints, including noninferiority in visual outcomes compared to on-label aflibercept,17,18 as well as greater than 85% treatment burden reduction and 64% supplemental injection-free rate at 6 months.
Axpaxli (OTX-TKI; Ocular Therapeutix) is an intravitreal implant with a poly(ethylene glycol) (PEG) hydrogel delivery system that delivers axitinib, a small-molecule TKI with pan-VEGF inhibitory properties, over 6 to 12 months. A recent phase 1 study disclosed encouraging initial safety, biologic activity, and durability data of Axpaxli in individuals with nAMD,19 and a pivotal phase 3 clinical trial is currently under way.
KSI-301 (tarcocimab tedromer; Kodiak Sciences) is a humanized anti-VEGF monoclonal antibody conjugated to a high molecular weight phosphorylcholine-based biopolymer. It is being assessed in diabetic retinopathy, diabetic macular edema, macular edema due to vein occlusion, and nAMD.20,21 Kodiak Sciences is also developing KSI-501ABC, a bispecific antibody built on the company’s Antibody Biopolymer Conjugate (ABC) platform with inhibition of both VEGF-A and IL-6, which is currently in a phase 1 study for diabetic macular edema.22
Re-Vana Therapeutics has pioneered the development of an in situ forming photo-crosslinkable gel system (OcuLief), and a preformed photo-crosslinked implant (EyeLief). These innovations have demonstrated efficacy in releasing anti-VEGF drugs, specifically bevacizumab, for a duration of up to 3 months.23 However, photosensitive hydrogels may risk compromising protein therapeutic integrity.24
Reservoirs for Intravitreal Delivery
Long-acting reservoirs aim to reduce intervention frequency and extend the effectiveness of existing anti-VEGF medications. The ranibizumab port delivery system (Susvimo; Genentech) is a replenishable intraocular reservoir that necessitates merely 2 “fills” annually to achieve efficacy comparable to monthly ranibizumab administration for up to 2 years.25 Susvimo was FDA approved for the treatment of nAMD in 2021. There is a black-box warning regarding the heightened risk of endophthalmitis in approximately 2% of participants after surgical implantation of the reservoir; more recently, Genentech declared a voluntary recall of the ranibizumab port delivery system and temporarily halted new implantations due to reported incidents of septum dislodgement. The current commercial implant supply is undergoing rigorous quality control assessments.
Suprachoroidal Delivery of Small-molecule Suspensions
Small-molecule suspensions have historically been limited in their use intravitreally due to potential for “snow globe” effects; however, the innovation of delivery to the suprachoroidal space (SCS) provides an opportunity for their use in nAMD with targeting of affected chorioretinal tissues and potential sustained drug-release kinetics.26 Microneedles designed to access the SCS facilitate effective drug delivery to the chorioretina without penetrating the vitreous, minimizing off-target effects and potentially enhancing safety.27,28
CLS-AX (Clearside Biomedical) is a suprachoroidal injection of axitinib under development as a potential semiannual therapeutic option for nAMD. Outcomes from the phase 1/2a OASIS trial demonstrated positive safety, durability, and clinical effect of CLS-AX in nAMD.29 After 1 CLS-AX injection of 0.5 mg or 1.0 mg, subjects demonstrated stable visual and anatomic outcomes with minimal supplemental aflibercept treatment up to 6 months. Clearside Biomedical recently announced completion of recruitment for its phase 2b ODYSSEY trial comparing CLS-AX every 24 weeks to bimonthly intravitreal aflibercept.30
Gene Therapy Biofactories
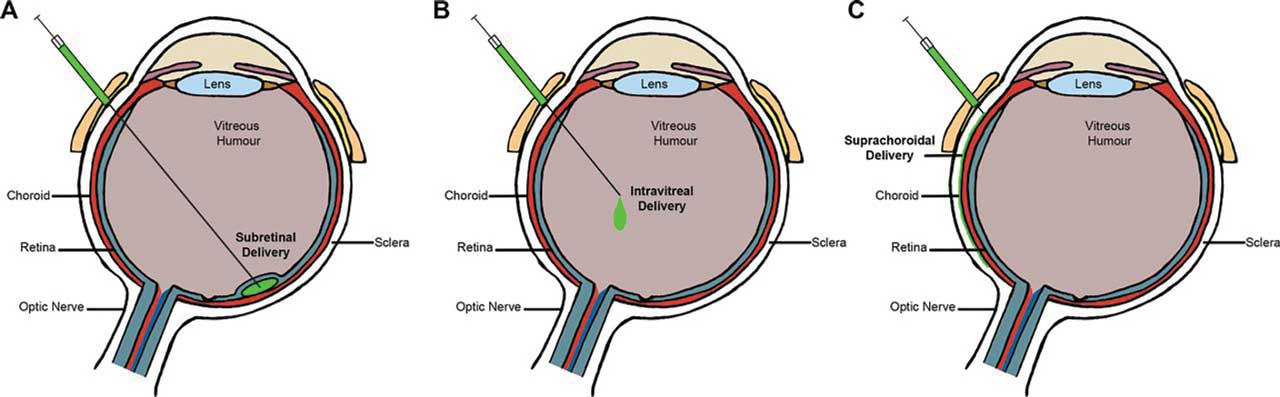
Retina has been at the forefront of gene therapy in medicine since the FDA clearance of voretigene neparvovec-rzyl (Luxturna; Spark Therapeutics) in December 2017. The ability of therapeutic genes to continuously produce desired proteins, such as anti-VEGF, holds promise for reducing the treatment burden of frequent intravitreal injections in nAMD. While subretinal injection is the standard for ocular gene therapy, alternative routes with suprachoroidal and intravitreal delivery are being explored to avoid complications associated with pars plana vitrectomy and retinotomy (Figure 4).31
ABBV-RGX-314 is a novel gene therapy intervention being developed by RegenxBio and AbbVie that delivers a transgene encoding a ranibizumab-like anti-VEGF monoclonal antibody fragment through an adeno-associated virus (AAV) 8 vector (Figure 5). A phase 1/2a study reported positive safety and efficacy results with the subretinal delivery of ABBV-RGX-314 in nAMD.32 The ongoing phase 2b/3 ATMOSPHERE and ASCENT trials are assessing 2 dose levels of subretinal ABBV-RGX-314 against monthly ranibizumab and bimonthly aflibercept, respectively.33, 34

Simultaneously, RegenxBio is evaluating the suprachoroidal delivery, via Clearside’s SCS Microinjector, of ABBV-RGX-314 in the phase 2 AAVIATE trial. Interim data demonstrated an 80% reduction in annualized injection rate and 50% injection-free rate up to 6 months following a single suprachoroidal injection of ABBV-RGX-314.35 Furthermore, the therapy was well tolerated in all 3 dose levels with no drug-related serious adverse events.
Ixoberogene soroparvovec (ixo-vec, formerly ADVM-022; Adverum Biotechnologies) is a single-use intravitreal gene therapy that employs the AAV2.7m8 capsid to induce endogenous aflibercept production. The phase 1 OPTIC study supported the safety of ixo-vec in nAMD and displayed up to a 98% reduction in annualized anti-VEGF injection burden and 80% injection-free rate.36 The ongoing phase 2 LUNA trial is investigating 2 doses of ixo-vec in combination with enhanced corticosteroid prophylaxis.
Lastly, 4D-150 (4D Molecular Therapeutics) is a single-use, low-dose intravitreal gene therapy utilizing an evolved vector, R100, and a transgene cassette that expresses both aflibercept and a VEGF-C inhibitory RNAi. Interim results from the phase 1/2 PRISM trial demonstrated the safety and tolerability of 4D-150, in addition to up to an 89% reduction in annualized anti-VEGF injection rate in nAMD subjects.37, 38
Conclusion
The innovative approaches discussed here showcase the evolving landscape of sustained-release technologies for nAMD treatment, with the common goal of reducing treatment burden and improving patients’ quality of life and vision preservation. Ongoing randomized, clinical trials and real-world data will be crucial to fully assess the safety and effectiveness of these therapies in diverse clinical settings. RP
References
- Campbell RJ, Bronskill SE, Bell CM, Paterson JM, Whitehead M, Gill SS. Rapid expansion of intravitreal drug injection procedures, 2000 to 2008: a population-based analysis. Arch Ophthalmol. 2010;128(3):359-362. doi:10.1001/archophthalmol.2010.19
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. doi:10.1016/j.ophtha.2019.04.017
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration [published correction appears in Ophthalmology. 2013;120(1):209-10]. Ophthalmology. 2012;119(12):2537-2548. doi:10.1016/j.ophtha.2012.09.006
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Maguire MG, Martin DF, et al. Five-year outcomes with anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123(8):1751-1761. doi:10.1016/j.ophtha.2016.03.045
- CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. doi:10.1056/NEJMoa1102673
- Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti–vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122(4):803-808. doi:10.1016/j.ophtha.2014.11.018
- Khanani AM, Skelly A, Bezlyak V, Griner R, Torres LR, Sagkriotis A. SIERRA-AMD: a retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4(2):122-133. doi:10.1016/j.oret.2019.09.009
- Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4(1):19-30. doi:10.1016/j.oret.2019.05.017
- Moshfeghi AA, Pitcher JD, Lucas G, Boucher N, Saroj N. Visual acuity outcomes in patients receiving frequent treatment of neovascular age-related macular degeneration in clinical practice. J Vitreoretin Dis. 2020;5(3):221-226. doi:10.1177/2474126420960896
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729-740. doi:10.1016/S0140-6736(22)00010-1
- Penha FM, Masud M, Khanani ZA, et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int J Retina Vitreous. 2024;10(1):5. Published 2024 Jan 17. doi:10.1186/s40942-024-00525-9
- Baumal CR, Sørensen TL, Karcher H, et al. Efficacy and safety of brolucizumab in age-related macular degeneration: a systematic review of real-world studies. Acta Ophthalmol. 2023;101(2):123-139. doi:10.1111/aos.15242
- Wykoff CC, Matsumoto H, Barakat MR, et al. Retinal vasculitis or vascular occlusion after brolucizumab for neovascular age-related macular degeneration: a systematic review of real-world evidence. Retina. 2023;43(7):1051-1063. doi:10.1097/IAE.0000000000003769
- Spitzer MS. Intravitreal aflibercept 8 mg injection in patients with neovascular age-related macular degeneration: 48-week results from the Phase 3 PULSAR trial. Invest Ophthalmol Vis Sci. 2023;64:461.
- Chaudhary V, Barbosa J, Lam WC, Mak M, Mavrikakis E, Mohaghegh P SM. Ozurdex in age-related macular degeneration as adjunct to ranibizumab (The OARA Study). Can J Ophthalmol. 2016;51(4):302-305. doi:10.1016/j.jcjo.2016.04.020
- Ranchod TM, Ray SK, Daniels SA, Leong CJ, Ting TD, Verne AZ. LuceDex: a prospective study comparing ranibizumab plus dexamethasone combination therapy versus ranibizumab monotherapy for neovascular age-related macular degeneration. Retina. 2013;33(8):1600-1604. doi:10.1097/IAE.0b013e318285cb71
- Abbey AM, Patel SS, Barakat M, et al. The DAVIO trial: a phase 1, open-label, dose-escalation study of a single injection of EYP-1901 (vorolanib in Durasert platform) demonstrating reduced treatment burden in wet age-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64:931.
- EyePoint Pharmaceuticals announces positive topline data from the phase 2 DAVIO 2 trial of EYP-1901 in wet AMD achieving all primary and secondary endpoints. News release. December 4, 2023. Accessed February 21, 2024. https://investors.eyepointpharma.com/news-releases/news-release-details/eyepoint-pharmaceuticals-announces-positive-topline-data-phase-2
- Moshfeghi AA, Khanani AM, Eichenbaum DA, et al. US phase 1 study of intravitreal axitinib implant (OTX-TKI) for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64:936.
- Kodiak Sciences announces topline results from its phase 3 studies of tarcocimab tedromer in neovascular age-related macular degeneration and diabetic macular edema and provides update on tarcocimab development program. News release. July 24, 2023. Accessed February 21, 2024. https://ir.kodiak.com/news-releases/news-release-details/kodiak-sciences-announces-topline-results-its-phase-3-studies/
- Regillo C, Ehrlich JS, Janer D, et al. Efficacy, durability, and safety of KSI-301 antibody biopolymer conjugate in wet AMD — Year 1 primary endpoint results from the pivotal DAZZLE study. Invest Ophthalmol Vis Sci. 2022;63:3122.
- Kodiak Sciences announces upcoming presentation of first-time results of KSI-501ABC phase 1 study at the Angiogenesis, Exudation, and Degeneration 2024 Virtual Meeting. News release. January 30, 2024. Accessed February 21, 2024. https://ir.kodiak.com/news-releases/news-release-details/kodiak-sciences-announces-upcoming-presentation-first-time/
- Wells LA, Furukawa S, Sheardown H. Photoresponsive PEG-anthracene grafted hyaluronan as a controlled-delivery biomaterial. Biomacromolecules. 2011;12(4):923-932. doi:10.1021/bm101233m
- McAvoy K, Jones D, Thakur RRS. Synthesis and characterization of photocrosslinked poly(ethylene glycol) diacrylate implants for sustained ocular drug delivery. Pharm Res. 2018;35(2):36. doi:10.1007/s11095-017-2298-9
- Holekamp NM, Campochiaro PA, Chang MA, et al. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307. doi:10.1016/j.ophtha.2021.09.016
- Wan CR, Muya L, Kansara V, Ciulla TA. Suprachoroidal delivery of small molecules, nanoparticles, gene and cell therapies for ocular diseases. Pharmaceutics. 2021;13(2):288. doi:10.3390/pharmaceutics13020288
- Goldstein DA, Ciulla TA. Suprachoroidal delivery of suspensions of tyrosine kinase inhibitor, complement inhibitor, and corticosteroid: preclinical and clinical correlates. Invest Ophthalmol Vis Sci. 2020;61:2898.
- Muya L, Kansara V, Cavet ME, Ciulla T. Suprachoroidal injection of triamcinolone acetonide suspension: ocular pharmacokinetics and distribution in rabbits demonstrates high and durable levels in the chorioretina. J Ocul Pharmacol Ther. 2022;38(6):459-467. doi:10.1089/jop.2021.0090
- Marcus DM, Hu A, Barakat M, et al. Safety and tolerability study of suprachoroidal injection CLS-AX in neovascular AMD patients with persistent activity following anti-VEGF therapy (OASIS, NCT04626128; extension study NCT NCT05131646). Invest Ophthalmol Vis Sci. 2023;64:728.
- Clearside Biomedical completes recruitment in ODYSSEY phase 2b clinical trial of CLS-AX in wet AMD. News release. November 1, 2023. Accessed February 21, 2024. https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-completes-recruitment-odyssey-phase-2b
- Peng Y, Tang L, Zhou Y. Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58(4):217-226. doi:10.1159/000479157
- Safety and tolerability of RGX-314 (investigational product) gene therapy for neovascular AMD trial. Clinicaltrials.gov identifier: NCT03066258. Updated May 16, 2023. Accessed February 21, 2024. https://www.clinicaltrials.gov/study/NCT03066258?Tab=results
- Pivotal 1 study of RGX-314 gene therapy in participants with nAMD. Clinicaltrials.gov identifier: NCT04704921. Updated May 22, 2023. Accessed February 21, 2024. https://clinicaltrials.gov/study/NCT04704921
- Pivotal 2 study of RGX-314 gene therapy in participants with nAMD. Clinicaltrials.gov identifier: NCT05407636. Updated August 21, 2023. Accessed February 21, 2024. https://clinicaltrials.gov/study/NCT05407636
- Regenxbio announces positive interim data from phase II AAVIATE trial of ABBV-RGX-314 for the treatment of wet AMD using suprachoroidal delivery. News release. January 16, 2024. Accessed February 21, 2024. https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-positive-interim-data-phase-ii-aaviater/
- Khanani AM, Boyer DS, Wykoff CC, et al. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): a prospective, two-year, multicenter phase 1 study. EClinicalMedicine. 2023;67:102394. doi:10.1016/j.eclinm.2023.102394
- Khanani AM, Hershberger VS, Kay CN, et al. Interim results for the phase 1/2 PRISM trial evaluating 4D-150, a dual-transgene intravitreal genetic medicine in individuals with neovascular (wet) age-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64:5055.
- 4DMT presents positive interim data from randomized phase 2 PRISM clinical trial of intravitreal 4D-150 demonstrating favorable tolerability & clinical activity in wet AMD. News release. February 3, 2024. Accessed February 21, 2024. https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-presents-positive-interim-data-randomized-phase-2-prism/










