Age-related macular degeneration (AMD) is the fourth leading cause of blindness in older adults and is estimated to affect 8.7% of the global population.1,2 The neovascular, or exudative (“wet”), form of the disease currently requires long-term treatment with intravitreal anti–vascular endothelial growth factor (VEGF) pharmacotherapy, resulting in a high treatment and office visit burden experienced by patients and their caregivers.3
Financial costs of neovascular AMD (nAMD) are significant, ranging between $8,814 and $23,400 per year, or $32,491 to $70,200 after 3 years of treatment, according to recent analyses.4 Accordingly, several gene therapies are being explored in clinical testing as potential options to help mitigate the need for frequent injections and potentially curb long-term costs associated with chronic intravitreal injection (IVI) therapy.
Recently, there have been several promising updates from clinical trial programs exploring the role of various gene therapies in nAMD. As gene therapy research continues to expand within the nAMD space, major factors should be considered, including long efficacy and durability of the therapeutic payload, safety profile, and overall expenditures to the health care system.
Delivery of Gene Therapy
Gene therapy research in ophthalmology is exploring 3 potential delivery routes for treatment of nAMD, which have different considerations regarding safety and efficacy (Figure 1). Intravitreal delivery has the potential advantage of convenient administration within the office setting. However, it lacks efficient delivery and transduction due to ocular barriers, such as potential neutralization due to preexisting antibodies.5 Intravitreal delivery of pharmacotherapeutics can also be associated with intraocular inflammation, retinal detachments, endophthalmitis, and intraocular pressure elevation.6
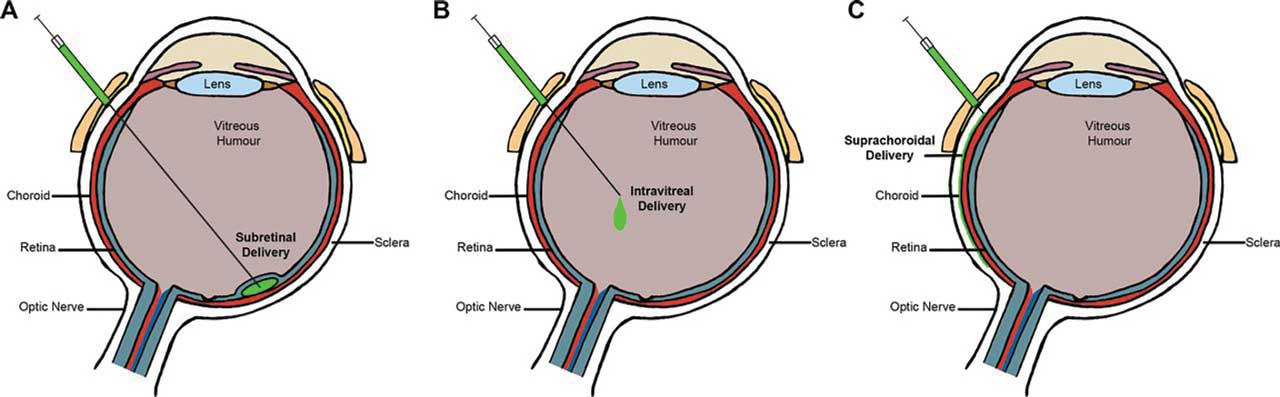
The subretinal approach via the transvitreal route directly delivers vectors to the retinal pigmented epithelial and retinal layer, a space considered more immune privileged than the vitreous, with mechanisms significantly reducing inflammation risk.7 The subretinal approach also avoids potential neutralizing antibodies, which increases transduction and protein expression.8 However, a disadvantage of this route is that it requires a surgical approach with concomitant pars plana vitrectomy in the operating room.
Lastly, the suprachoroidal injection approach delivers the gene therapy payload via the potential space between the sclera and the choroid (suprachoroidal space, or SCS). This is a potentially favored injection site for gene therapy because it may reduce the likelihood of eliciting an immunologic response due to its unique location with respect to the blood-retina barrier while simultaneously permitting adequate gene expression to control the nAMD disease process.9 Surgical access to the SCS can be facilitated by creation of a sclerotomy, followed by introduction of a catheter or cannula. Separately, and potentially of greater interest, is for suprachoroidal gene therapy to be delivered in the clinic setting by utilizing hypodermic needles or hollow microneedles. What follows is an update on the status of some of promising gene therapies.
ADVM-022/Ixo-vec
ADVM-022 (Adverum Biotechnologies), now known as Ixo-vec (ixoberogene soroparvovec), is a potential in-office intravitreal gene therapy treatment option for nAMD. The treatment consists of a recombinant adeno-associated virus (AAV) using the AAV.7m8 capsid to deliver a codon-optimized cDNA of the anti-VEGF aflibercept protein.10
The 3-year outcomes for the treatment have been reported from the phase 1 dose-escalation OPTIC trial, which included 2 treatment arms: 2x10¹¹ vg (vector genomes) and 6x10¹¹ vg. Adverum announced the 2x10¹¹ vg dose of Ixo-vec demonstrated safety and efficacy with an 84% reduction in annual anti-VEGF injections, with an impressive 53% of this cohort remaining injection-free over the entire 3-year period. Long-term production of aflibercept protein levels helped maintain best-corrected visual acuity (BCVA) and improved central subfield thickness (CST) measurements on ancillary optical coherence tomography (OCT) imaging. The mean BCVA change from baseline to last visit was +0.2 ETDRS letters, and the mean CST change from baseline to last visit was -92.9 μm.11
Participants from the OPTIC trial were then included in the LUNA trial, a randomized double-masked phase 2 trial.12 To improve safety and reduce intraocular inflammation, dosing was randomized between the 2x10¹¹ vg and a new, lower, 6x10¹⁰ vg dose. Additionally, 4 prophylactic steroid regimens were added to control intraocular inflammation, including topical difluprednate (Durezol; Novartis), dexamethasone intravitreal implant (Ozurdex; AbbVie), or a combination of either topical difluprednate or intravitreal dexamethasone implant with oral prednisone.13
Adverum reported that both dose levels (2x10¹¹ vg and 6x10¹⁰ vg) reduced treatment burden for patients while maintaining visual acuity and achieving anatomic (CST) endpoints. Patients had an annual reduction of anti-VEGF injection rates of 90% (n=19) at 6x10¹⁰ and 94% (n=20) at 2x10¹¹ at 26 weeks with Ixo-vec. In addition, Ixo-vec demonstrated an injection-free rate of 68% (n=19) at 6x10¹⁰ vg dose and 85% (n=20) at 2x10¹¹ vg dose. Visual acuity and anatomic endpoints were maintained at both dose levels. The mean BCVA change from baseline to last visit at a dose of 2x10¹¹ vg was -1.7 letters, and at a dose of 6x10¹⁰ vg the mean BCVA change was +0.5 letters. The mean CST change from baseline to last visit at a dosage of 2x10¹¹ was -16.4 μm, and at a dose of 6x10¹⁰, it was -7.9 μm. There was a greater CST reduction in patients who had a baseline CST greater than 300 μm which was maintained over the 26 weeks (-24.9 for patients with >300 μm and 3.4 for patients with ≤300 μm). Aflibercept protein expression levels aligned with those observed in the previous OPTIC trial. The visual acuity and anatomic (CST) endpoints shown in LUNA confirmed the activity observed in OPTIC.13
No serious adverse events (SAEs) related to Ixo-vec were reported in LUNA; all reported events were classified as mild to moderate. Dose-related anterior inflammation (primarily anterior chamber [AC] cells) and anterior pigmentary changes were the most common instances, which was consistent with the previous OPTIC trial. Adjunct intravitreal dexamethasone implant plus topical difluprednate drops was determined to be a promising prophylaxis treatment, as >90% of the study group on this dual therapy had minimal to no inflammation. Those with minimal inflammation were graded no higher than 0.5+ trace AC cells.12 However, there was no incremental benefit from oral corticosteroids or dexamethasone intravitreal implant (± oral corticosteroids). There was an improved inflammatory profile in the LUNA trial due to the prophylaxis treatment over the OPTIC 2x10¹¹ vg trial. In both doses of Ixo-vec the mean intraocular pressure (IOP) was stable.
RGX-314
RGX-314 (Regenxbio) is an adeno-associated virus serotype 8 (AAV8) gene therapy for nAMD, designed to express monoclonal antibody fragments that neutralize VEGF activity. ASCENT and ATMOSPHERE are ongoing randomized, partially masked, controlled studies examining the safety and efficacy of subretinal delivery of 2 different doses of RGX-314 against intravitreal injection of aflibercept (ASCENT) and ranibizumab (ATMOSPHERE).14,15 The main outcome measure will be a mean change in BCVA between the different treatment arms in each study.
Conversely, the AAVIATE study is evaluating a suprachoroidal injection approach for RGX-314. The interim 6-month results of the phase 2 trial were reported on January 16, 2024. This study examined 3 different dosages of RGX-314: dose 1 (2.5x10¹¹ genomic copies [GC]/eye), dose 2 (5.0x10¹¹ GC/eye), and dose 3 (1.0x10¹² GC/eye). RGX-314 reported that the drug was well tolerated in each dose cohort. There was a total of 15 adverse events, which were determined to be unrelated to the drug, and, importantly, no SAEs in 106 treated patients to date. The most common side effects were mild intraocular inflammation, conjunctival hyperemia, conjunctiva hemorrhage, episcleritis, and increased IOP, which was less common.16
Over the 6-month period, patients treated with RGX-314 demonstrated stable functional and anatomic endpoints. In addition, the RGX-314 study group showed a decrease in injection burden, ranging from a 63.8% to 84.7% decrease in injections, depending on the dose cohort. Additionally, researchers included an additional cohort to determine if supplementing with prophylactic ocular steroids could reduce complications with intraocular inflammation. Patients receiving dose level 3 of RGX-314 were randomized to 1 of 2 steroid regimens; no cases in this cohort experienced intraocular inflammation.17
KH631
KH631 (Chengdu Origen and Vanotech) is a gene therapy undergoing phase 1 evaluation as an intravitreal recombinant AAV8 vector therapy. It carries a gene that expresses fusion VEGF receptor proteins with a high affinity for VEGF-A, VEGF-B, and placental growth factor (PlGF), which are all involved in angiogenesis.18 Preclinical studies looking at KH631 expression in primates with laser-induced choroidal neovascularization showed promising results, controlling severe inflammation with long-term transgene protein expression longer than 96 weeks.19
On November 11, 2023, Chengdu Origen and Vanotech announced that the first patient was to be dosed with the KH631 therapy. Phase 1 will enroll 25 patients in a dose-escalation study to determine the recommended dosage based on safety profile.20
4D-150
The advent of biotechnology and computational advances have allowed for a diverse array of new vectors to improve the safety and efficacy of gene therapy delivery. Methods such as capsid shuffling or error-prone PCR allow for directed evolution, developing a diverse array of synthetic capsid designs that may help reduce the risk of triggering immune-related inflammation, as well as the neutralization of preexisting antibodies. Additionally, such techniques may improve the transduction of the transgene into targeted cells of interest.21
R100 for 4D-150 (4DMT) has utilized directed evolution to create a synthetic AAV capsid, an intravitreal gene therapy option for nAMD. The capsid bypasses the inner limiting membrane, allowing for higher retinal transduction of its transgenes, expressing aflibercept and a VEGF-C inhibitory RNAi. These inhibit VEGF-A, VEGF-B, VGEF-C, and PlGF.
The therapy is currently being evaluated in the PRISM trial, an ongoing phase 2 study. It includes a cohort of severe nAMD patients (n=51) with a pre-existing high treatment burden for their disease. Individuals were placed in either the high-dosage (3.0x1010 vg/eye; n=20) or low-dosage (1.0x1010 vg/eye; n=21) treatment arm, with an additional 2 mg aflibercept control cohort (n=10) receiving treatment every 8 weeks. All patients were started on a 20-week tapering course of prophylactic topical corticosteroids.
Interim data at week 24 — released on February 3, 2024 — demonstrated that BCVA was stable throughout all 3 treatment arms. Compared to the baseline visit, weeks 20 and 24 averages showed an increase of 1.8 EDTRS letters gained in the low-dosage arm and a slight average decrease of 1.8 EDTRS letters lost in the high-dosage arm. CST was also evaluated, showing aflibercept had the highest variability, with peaks near the end of each 8-week treatment and valleys near the midpoint between injection intervals. In contrast, high-dose 4D-150 showed significantly reduced variability, suggesting an underlying continuous sustainable release of endogenous anti-VEGF. The 24-week landmark analysis also revealed 89% and 85% reductions in the average number of yearly injections for high and low doses of 4D-150, respectively.22
Furthermore, 4D-150’s high-dose and low-dose arms demonstrated good safety profiles through the 48-week visit without significant inflammation or drug-related adverse events. During the week 16 visit from the low dosage cohort, 1 individual developed 1+ anterior cells, which resolved upon the next visit with a temporary increase in tropical corticosteroid dosage and later tapered off the steroid drops by week 26.
EXG102-031
EXG102-031 (Exegenesis Bio) is a subretinal injection of an rAAV-based gene therapy containing a transgene coding for a therapeutic fusion protein. Unlike other therapies, this gene product targets all subtypes of VEGF and angiopoietin-2 (ANG2). EXG102-031 is undergoing phase 1 trials to determine its safety and tolerability profile. Exegenesis Bio aims to enroll 6 participants for this open-label, multiple-cohort, dose-escalation study.23
Concerns Regarding Gene Therapy
Although gene therapy is a potential exciting treatment option for nAMD, several concerns regarding safety and efficacy, as well as cost, for these gene therapies need to be considered. While it is true that retinal gene therapies have the potential to offer a “one-and-done” treatment regimen via endogenously producing anti-VEGF protein mediated through viral vectors, this concept warrants careful consideration. Unlike in other fields of medicine with recently approved gene therapies — or even within ophthalmology with the introduction of voretigene neparvovec-rzyl (Luxturna; Spark Therapeutics), where treatment is replacing a missing or defective protein — gene therapies for nAMD would mark the first time for a “gain of function” option. With this potential, the concern comes that once the switch has been turned “on,” there are no readily accessible methods to deactivate the newly introduced expressed protein. What type of long-term sequelae may that carry 5, 10, or 20 years down the road?
Additionally, safety is always a paramount concern, especially regarding the potential for inducing intraocular inflammation. Chronic ocular inflammation may develop into secondary adverse events, including IOP elevation, steroid-induced cataracts, and uveitic macular edema.24 Given the risk of gene therapy–induced ocular inflammation, there has been an emphasis on the use of prophylactic steroid regimens with either intravitreal or topical therapy in the series of trials mentioned in this article. For example, in the OPTIC trial, the higher dose (6x10¹¹ vg/eye) of Ixo-vec was too high and induced inflammation. Ten out of the 15 patients who received the higher dose required chronic long-term steroid therapy. Additionally, in the parallel INFINITY trial of Ixo-vec for diabetic macular edema, side effects including hypotony, choroidal, and vision loss were reported with the higher 6x10¹¹ vg/eye dose.25 In response, the subsequent LUNA trial for Ixo-vec included an optimized prophylactic steroid regimen. Lowering the dose and starting the patient on a prophylactic steroid regimen can be the key to safely administering a gene therapy product. That being stated, the long-term ramifications are unknown. Might gene therapy potentially set an at-risk eye down a path of chronic uveitis that becomes challenging to manage with local or systemic therapies?
It is also unclear whether the balance of treatment will head toward longer-lasting anti-VEGF therapies or a singular one-and-done gene therapy treatment. Assuming there is a future with both options available, a head-to-head study between the 2 may be considered to compare drug safety and efficacy, long-term vision outcomes, quality of life, and cost-effectiveness. As a cost reference, Luxturna was estimated to be $850,000 for bilateral treatment of Leber congenital amaurosis.26 Although gene therapy comes with a substantial upfront fee, it may be offset by improvement in the patient’s quality of life and reduced treatment burden and downstream costs to the payer system.
Conclusion
In recent months, we have seen numerous promising interim updates from some of the lead gene therapy pipeline candidates in retina. These results have supported previous findings of long-term efficacy and durability; however, safety continues to be a challenging obstacle, especially the risk of treatment-induced ocular inflammation. Further studies and continued research will be necessary to provide physicians and patients the necessary confidence to consider these potential new treatment options if they do become available. Nevertheless, this is an exciting time, because gene therapy has the promise to disrupt our current injection treatment paradigm and remedy the treatment burden many of our patients currently face.RP
Hear discussion of this article on the Retina Podcast at www.retinapodcast.com.
References
1. Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila). 2017;6(6):493-497. doi:10.22608/APO.2017251
2. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study [published correction appears in Lancet Glob Health. 2021 Apr;9(4):e408]. Lancet Glob Health. 2021;9(2):e144-e160. doi:10.1016/S2214-109X(20)30489-7
3. Reitan G, Kjellevold Haugen IB, Andersen K, Bragadottir R, Bindesbøll C. Through the eyes of patients: understanding treatment burden of intravitreal anti-VEGF injections for nAMD patients in Norway. Clin Ophthalmol. 2023;17:1465-1474. doi:10.2147/OPTH.S409103
4. Geng C. Macular degeneration injections: does Medicare cover them? Medical News Today. April 25, 2023. Accessed March 130, 2024. https://www.medicalnewstoday.com/articles/does-medicare-pay-for-macular-degeneration-injections
5. Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22(2):116-126. doi:10.1038/gt.2014.115
6. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond). 2013;27(7):787-794. doi:10.1038/eye.2013.107
7. Chen M, Luo C, Zhao J, Devarajan G, Xu H. Immune regulation in the aging retina. Prog Retin Eye Res. 2019;69:159-172. doi:10.1016/j.preteyeres.2018.10.003
8. Desrosiers M, Dalkara D. Neutralizing antibodies against adeno-associated virus (AAV): measurement and influence on retinal gene delivery. Methods Mol Biol. 2018;1715:225-238. doi:10.1007/978-1-4939-7522-8_16
9. Wu KY, Fujioka JK, Gholamian T, Zaharia M, Tran SD. Suprachoroidal injection: a novel approach for targeted drug delivery. Pharmaceuticals (Basel). 2023;16(9):1241. doi:10.3390/ph16091241
10. Khanani AM, Boyer DS, Wykoff CC, et al. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): a prospective, two-year, multicentre phase 1 study. EClinicalMedicine. 2024;67:102394-102394. doi:https://doi.org/10.1016/j.eclinm.2023.102394
11. Adverum Biotechnologies announces 3-year efficacy and safety results from the OPTIC extension study in patients with wet AMD at AAO 2023. News release. November 4, 2023. Accessed March 13, 2024. https://investors.adverum.com/news/news-details/2023/Adverum-Biotechnologies-Announces-3-Year-Efficacy-and-Safety-Resultsfrom-the-OPTIC-Extension-Study-in-Patients-with-Wet-AMD-at-AAO-2023/default.aspx
12. Adverum Biotechnologies announces positive aflibercept protein level data from the LUNA phase 2 trial. News release. September 26, 2023. Accessed March 12, 2024. https://investors.adverum.com/news/news-details/2023/Adverum-Biotechnologies-Announces-Positive-Aflibercept-Protein-Level-Data-from-the-LUNA-Phase-2-Trial/default.aspx
13. Adverum Biotechnologies announces positive preliminary efficacy and safety data from LUNA phase 2 trial of Ixo-vec in patients with wet AMD. EIN News. February 8, 2024. Accessed March 13, 2024. https://www.einnews.com/pr_news/687132027/adverum-biotechnologies-announces-positive-preliminary-efficacy-and-safety-data-from-luna-phase-2-trial-of-ixo-vec-in-patients-with-wet-amd
14. Pivotal 2 study of RGX-314 gene therapy in participants with nAMD (ASCENT). ClinicalTrials.gov identifier: NCT05407636. Updated February 26, 2024. Accessed March 13, 2024. https://www.clinicaltrials.gov/study/NCT05407636
15. Pivotal 1 study of RGX-314 gene therapy in participants with nAMD (ATMOSPHERE). ClinicalTrials.gov identifier: NCT04704921. Updated March 4, 2024. Accessed March 13, 2024. https://clinicaltrials.gov/study/NCT04704921
16. Charters L, Boyer D. ASRS 2023: AAVIATE interim safety study 6-month results underscore treatment safety with suprachoroidally injected ABBV-RGX-314 for NAMD. Modern Retina. July 30, 2023. Accessed March 13, 2024. https://www.modernretina.com/view/asrs-2023-aaviate-interim-safety-study-6-month-results-underscore-treatment-safety-with-suprachoroidally-injected-abbv-rgx-314-for-namd
17. RGX-314 gene therapy administered in the suprachoroidal space for participants with neovascular age-related macular degeneration (nAMD) (AAVIATE). ClinicalTrials.gov Identifier: NCT04514653. Updated May 22, 2023. Accessed March 13, 2023. https://clinicaltrials.gov/study/NCT04514653
18. Safety and tolerability of KH631 gene therapy in participants with neovascular age-related macular degeneration. ClinicalTrials.gov identifier: NCT05657301. Updated February 26, 2024. Accessed March 13, 2024. https://www.clinicaltrials.gov/study/NCT05657301
19. Ke X, Jiang H, Li Q, et al. Preclinical evaluation of KH631, a novel rAAV8 gene therapy product for neovascular age-related macular degeneration. Mol Ther. 2023;31(11):3308-3321. doi:10.1016/j.ymthe.2023.09.019
20. Chengdu Origen and Vanotech announce first patient dosed in VAN-2201 phase 1 trial of gene therapy for wet age-related macular degeneration. BioSpace. November 20, 2023. Accessed March 13, 2024. https://www.biospace.com/article/releases/chengdu-origen-and-vanotech-announce-first-patient-dosed-in-van-2201-phase-1-trial-of-gene-therapy-for-wet-age-related-macular-degeneration/
21. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358-378. doi:10.1038/s41573-019-0012-9
22. 4D Molecular Therapeutics. 4DMT presents positive interim data from randomized phase 2 PRISM clinical trial of intravitreal 4D-150 demonstrating favorable tolerability and clinical activity in wet AMD. News release. February 3, 2024. Accessed March 13, 2024. https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-presents-positive-interim-data-randomized-phase-2-prism
23. A study of EXG102-031 in patients with wAMD (Everest). ClinicalTrials.gov identifier: NCT05903794. Updated February 26, 2024. Accessed March 10, 2024. https://www.clinicaltrials.gov/study/NCT05903794
24. Miller JR, Hanumunthadu D. Inflammatory eye disease: an overview of clinical presentation and management. Clin Med (Lond). 2022;22(2):100-103. doi:10.7861/clinmed.2022-0046
25. Yiu GC, Khanani AM, Pepple KL. Current challenges in gene therapy trials. Retinal Physician. March 1, 2023. Accessed March 13, 2024. https://retinalphysician.com/issues/2023/march/current-challenges-in-gene-therapy-trials/
26. Kaiser PK. Gene therapy’s price tag. Retinal Physician. March 1, 2023. Accessed March 13, 2024. https://retinalphysician.com/issues/2023/march/upfront-gene-therapys-price-tag/












