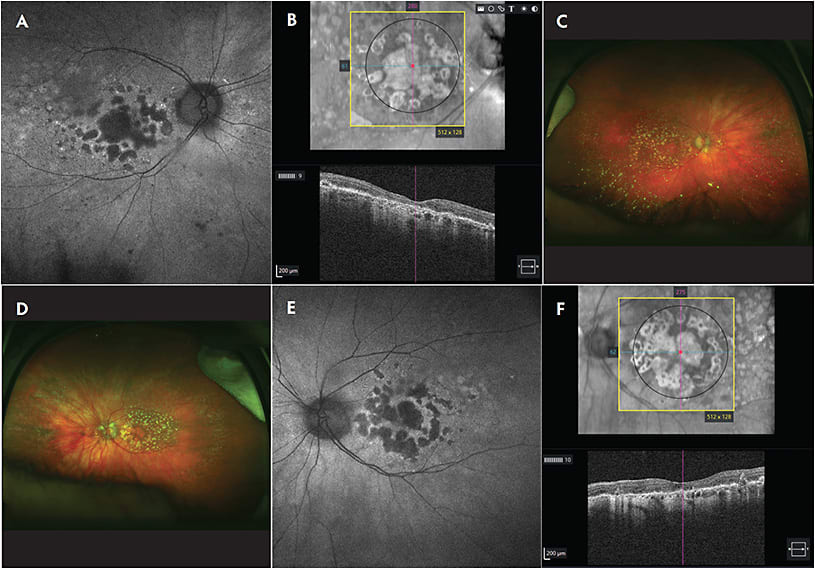
The development of geographic atrophy (GA) is hypothesized to be the culmination of oxidative stress, genetic predisposition, and environmental influences. The accumulation of cellular byproducts from these processes triggers inflammation and cell death (Figure 1) through various pathways, including the complement cascade. The diagnosis of GA has profound implications for the longevity of patients’ visual acuity, and therefore GA treatment warrants continuous investigation to improve visual and anatomic outcomes for these patients. Current emerging treatment strategies for GA and dry age-related macular degeneration (AMD) include neuroprotection and oxidative stress reduction, inhibition of toxic byproduct accumulation, visual cycle modulation, cell therapy, and suppression of inflammation, optogenetics, photobiomodulation, and retinal prosthesis, among other novel therapies.
ANTIOXIDANT AGENTS
There is emerging evidence regarding the important role of retinal mitochondrial dysfunction in AMD. At the cellular level, mitochondrial dysfunction leads to loss of ATP production, increased levels of reactive oxygen species (ROS), calcium dysregulation, and ultimately cell death. Multiple risk factors associated with the development of AMD through mitochondrial dysfunction include cigarette smoke exposure, lipofuscin accumulation within the RPE, and complement dysregulation. Additionally, genetic mitochondrial disorders such as maternally inherited diabetes and deadness and mitochondrial encephalopathy lactic acidosis, and stroke-like episodes often result in GA development, further underscoring the role of mitochondrial dysfunction in the pathophysiology of GA.
Elamipretide (Stealth Biotherapeutics)1,2 is a tetrapeptide that targets cardiolipin in mitochondria, reducing ROS production. Preclinical models of AMD have demonstrated the potential therapeutic benefit of elampretide, with evidence of reversed established vision loss and clearance of sub-RPE deposits in high-fat diet–fed APOE4 mouse models of dry AMD.3 The ReCLAIM study was a phase 1, single-site, open-label clinical study that evaluated the safety and tolerability of subcutaneous elampretide in subjects with dry AMD.4 Outcomes of this trial demonstrated that elampretide is safe and generally well tolerated in patients with dry AMD with noncentral geographic atrophy (NCGA) and high-risk drusen without GA.
The ReCLAIM-2 study evaluated the safety, efficacy, and pharmacokinetics of elampretide in patients with AMD with NCGA. Although the study did not meet its primary endpoints of mean change in low-luminance visual acuity (LLVA) and GA progression, there was appreciable categorical improvement in LLVA with more than 15% of patients treated with elampretide gaining 2+ lines of vision by week 48. Elamipretide-treated patients also demonstrated enhanced ellipsoid zone preservation through reduction of progressive attenuation.5
Risuteganib (Allegro Ophthalmics) is an anti-integrin small peptide molecule that downregulates oxidative stress. The safety and efficacy of intravitreal risuteganib for nonexudative AMD was assessed in a multicenter, phase 2a randomized clinical trial. Patients with nonexudative AMD and Early Treatment Diabetic Retinopathy Study (ETDRS) best corrected visual acuity (BCVA) between 20/40 and 20/200 were randomized to intravitreal 1.0 mg risuteganib or sham injection.6 At the 16-week time point, patients in the treatment group received a second 1.0 mg dose, and the placebo group crossed over to receive a 1.0 mg dose of risuteganib and were evaluated at the 28-week time point. The primary endpoint was proportion of subjects with 8 letters ETDRS or more BCVA gain from baseline to the 28-week time point in the treatment group vs from baseline to the 12-week time point in the placebo group; this endpoint was met by 48% of the risuteganib group and 7% of the placebo group.6 Additionally, in the treatment arm, 20% of study participants gained 15 letters or more at 28 weeks, whereas no patients in the sham group at 12 weeks achieved this gain in VA. There was a significant correlation between BCVA and color vision measures. All color vision metrics demonstrated a trend toward improvement in risuteganib responders (BCVA letter gain ≥8 letters) and no change in the nonresponders, with significant differences between the risuteganib and control group and between responders and nonresponders. Risuteganib responders also showed improvements in microperimetry measures, while nonresponders worsened during the time period of the study.7
Risuteganib demonstrated notable visual function improvements in patients with dry AMD, with no drug-related adverse events within the 32-week observation period. A phase 2b/3 clinical trial is planned.
ANTI-INFLAMMATORY AGENTS
Tetracyclines are a class of antibiotics that also exhibit anti-inflammatory properties. Low-dose oral doxycycline was investigated in the 24-month phase 2/3 TOGA clinical trial. This trial assessed the efficacy of doxycycline vs placebo in GA patients, with a primary outcome of rate of GA enlargement.8 Patients were randomized to 40 mg oral doxycycline or placebo daily. Eligible participants completed a 6-month observation phase, followed by a 24-month treatment phase, followed by an end-of-study visit. Participants were then randomized at 6 months in a 1:1 ratio to either 40 mg doxycycline or a placebo capsule to be taken once daily for 24 months. Preliminary data revealed low-dose doxycycline remained safe among the study cohort members, with a rate of wet AMD development of 2.4% and a rate of GA growth of 1.6 mm2.
INFLAMMASOME INHIBITION
Inflammasomes are molecular regulator complexes that help mediate inflammation and are composed of a central protein, an adaptor protein ASC, and a caspase-1 protein. Upon activation, caspase-1 induces maturation of proinflammatory cytokines and additional signaling that can lead to the pathology of inflammatory, autoimmune, and infectious disease. The role of inflammasomes, especially NLRP3, in the pathogenesis of chronic age-related eye diseases has been well studied.9 Experimental studies have revealed that the inhibition of inflammasomes generally helps to reduce the inflammation associated with these eye diseases, but a review of the role of inflammasomes in the pathology of eye disease is warranted at this time. Yerramothu et al reviewed the role of inflammasomes in the pathogenesis of several ocular diseases, including AMD.10 In AMD, the NLRP3 inflammasome is activated through many causative factors, including drusen components, RPE and complement proteins, oxidative stress and byproducts, and DNA.11-13 The deposition of drusen over the RPE results in the necrotic destruction of these cells and consequent activation of the NLRP3 inflammasome.14 A component of drusen, complement protein C1q, activates the NLRP3 inflammasome through phagolysosome activity.15
Therapies inhibiting NLRP3 inflammasome or the 2 cytokines activated by NLRP3 (IL-1β and IL-18) are currently being evaluated in preclinical programs. A fusion protein (rilonacept) developed by Regeneron Pharmaceuticals can bind to both IL-1α and IL-1β. Another inflammasome inhibitor under investigation is canakinumab (Novartis), a human immunoglobulin monoclonal antibody that specifically targets IL-1β.16 Several clinical trials have been undertaken to elucidate the efficacy of GSK1070806 (GlaxoSmithKline), an antibody directed against IL-18. There is currently limited information about the safety and efficacy of using this antibody systemically, but many of the trials are marked as completed, suggesting the systemic inhibition of IL-18 is tolerated in human subjects. Another therapeutic approach focuses on targeting inflammasome constituents to block the release of IL-1β and IL-18. The development of small-molecule inhibitors that directly target the components of the inflammasome may prove to be cheaper and less invasive modes of therapy than the larger biologics in use currently that focus on sequestering IL-1β after it has been secreted.
GLYCOPROTEIN SIGLECS
Siglecs are defined as primary drivers of immune response resolution, often composed of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that are implicated in cell signaling and endocytosis.17 AVD-104 (Aviceda) is an in vitro transcribed molecule that influences both complement and inflammatory pathways and has an emerging role in the discussion regarding GA management. AVD-104 targets Siglecs 7, 9, and 11 receptors on macrophages and demonstrates both in vitro and in vivo inhibition of complement and inflammatory pathways. This molecule has demonstrated potential for efficacy, safety, and an extended dosing regimen (>q12 weeks), and to reduce conversion to wet AMD. This work builds upon the positive findings from the Apellis pegcetacoplan 24-month study in GA,18 with a focus to build a product with an improved safety profile, reduced conversion to wet AMD, and a more convenient dosing regimen.
Aviceda recently completed both good laboratory practice (GLP) toxicology and in-vivo choroidal neovascularization (CNV) studies that revealed potential superiority in each of these areas. AVD-104 demonstrated safety in multiple animal models including nonhuman primates and rabbits. Each GLP toxicology study was evaluated in 40 animals each (total of 80 animals) with full toxicokinetic analyses and ocular/systemic safety evaluations. Three doses of AVD-104 (low, mid, high) were compared to control, with no reported ocular, systemic, or other severe adverse events reported. Additionally, AVD-104 also demonstrated the ability to reduce CNV lesion size and leakage comparable to aflibercept in a well-established, laser-induced CNV mouse model of choroidal neovascularization. These data suggest the potential of AVD-104 to prevent conversion of dry AMD to neovascular AMD in patients, in contrast to increased CNV conversion as seen with pegcetacoplan and avacincaptad.19
OPTOGENETICS
Optogenetics refers to the use of light and molecular genetics to modify the activity of live cells by expressing light-sensitive proteins, which cause neuronal cells to respond to light stimulation.20 GenSight Biologics is spearheading the development of the gene therapy GS030 consisting of 2 complementary proteins. One product encodes a photoactivatable channelrhodopsin protein that will be delivered through a modified AAV2 vector, while the second component consists of biomimetic goggles that stimulate the engineered retinal cells.21 The aforementioned vector introduces the gene encoding the photosensitive protein into the nucleus of the target cells of interest, specifically the retinal ganglion cells. This gene encodes a photoreceptor function to healthy and preserved retinal ganglion cells irrespective of any underlying genetic mutation causing photoreceptor degeneration. GenSight aims to investigate the potential for GS030 to treat GA after the conclusion of the PIONEER study in 2025. Applied Genetic Technologies Corporation (AGTC), Nanoscope Therapeutics, and Novartis also have promising early optogenetic programs for GA.
GENE THERAPY
GT005 (Novartis/Gyroscope Therapeutics) is an AAV-based gene therapy designed to rebalance an overactive complement system by expressing the complement factor I (CFI), a natural inhibitor of the complement system. The FOCUS phase 1/2 trial is evaluating the safety, dose response, and efficacy (anatomical and visual outcomes) of 3 doses of GT005 administered as a single subretinal injection in patients with macular atrophy due to AMD.22 Interim results of this study showed GT005 was well tolerated and yielded increases in expression of the CFI protein, as well as a 41% and 42% decrease in the levels of the Ba and C3 proteins in the vitreous, respectively.23 There was also a statistically significant correlation between increased CFI levels and decreases in Ba levels. GT005 is currently being evaluated in the phase 2 trials EXPLORE and HORIZON. EXPLORE is a trial studying the safety and efficacy of GT005 in patients with GA secondary to AMD who have rare variants of the CFI gene that is associated with low levels of CFI protein in their blood. HORIZON is a study evaluating the safety and efficacy of 2 doses of GT005 administered as a single subretinal injection and is currently enrolling up to 180 patients with GA secondary to AMD.24
CONCLUSIONS
Geographic atrophy secondary to AMD is a progressive ocular disease that leads to irreversible vision loss. Innovative treatments are required to reduce individual burden of disease as well as societal cost burden as the prevalence of AMD continues to rise with the aging population. The treatment strategies outlined demonstrate varying levels of success, and many of the ongoing clinical trials discussed offer promising preliminary results, suggesting that a paradigm shift in the treatment of GA may be on the horizon. RP
Editor’s note: This article is part of a special edition of Retinal Physician that was supported by Apellis Pharmaceuticals. Authors and editors maintained editorial control for all articles in this special edition.
REFERENCES
- Liu S, Soong Y, Seshan SV, Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol. 2014;306(9):F970-F980. doi:10.1152/ajprenal.00697.2013
- Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-2050. doi:10.1111/bph.12461
- Cousins SW, Saloupis P, Brahmajoti MV, Mettu PS. Mitochondrial dysfunction in experimental mouse models of subrpe deposit formation and reversal by the mito-reparative drug MTP-131 [ARVO abstract]. Invest Ophthalmol Vis Sci. 2016;57(12):2126.
- Mettu PS, Allingham MJ, Cousins SW. Phase 1 clinical trial of elamipretide in dry age-related macular degeneration and noncentral geographic atrophy: ReCLAIM NCGA study. Ophthalmol Sci. 2021;2(1):100086. doi:10.1016/j.xops.2021.100086
- Stealth BioTherapeutics announces data from the phase 2 ReCLAIM-2 study of elamipretide in geographic atrophy at the clinical trials at the summit meeting 2022. News release. May 23, 2022. Accessed December 20, 2022. https://www.prnewswire.com/news-releases/stealth-biotherapeutics-announces-data-from-the-phase-2-reclaim-2-study-of-elamipretide-in-geographic-atrophy-at-the-clinical-trials-at-the-summit-meeting-2022-301552607.html
- Boyer DS, Gonzalez VH, Kunimoto DY, et al. Safety and efficacy of intravitreal risuteganib for non-exudative AMD: a multicenter, phase 2a, randomized, clinical trial. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):327-335. doi:10.3928/23258160-20210528-05
- Lad EM, Boyer DS, Heier JS, et al. Color vision and microperimetry changes in nonexudative age-related macular degeneration after risuteganib treatment: exploratory endpoints in a multicenter phase 2a double-masked, randomized, sham-controlled, crossover clinical trial. Ophthalmic Surg Lasers Imaging Retina. 2022;53(8):430-438. doi:10.3928/23258160-20220725-02
- Rubner R, Li KV, Canto-Soler MV. Progress of clinical therapies for dry age-related macular degeneration. Int J Ophthalmol. 2022;15(1):157-166. Published 2022 Jan 18. doi:10.18240/ijo.2022.01.23
- Mugisho OO, Green CR. The NLRP3 inflammasome in age-related eye disease: evidence-based connexin hemichannel therapeutics. Exp Eye Res. 2022;215:108911. doi:10.1016/j.exer.2021.108911
- Yerramothu P, Vijay AK, Willcox MDP. Inflammasomes, the eye and anti-inflammasome therapy. Eye (Lond). 2018;32(3):491-505. doi:10.1038/eye.2017.241
- Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18(5):791-798. doi:10.1038/nm.2717
- Tseng WA, Thein T, Kinnunen K, et al. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(1):110-120. doi:10.1167/iovs.12-10655
- Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells--implications for age-related macular degeneration (AMD). Immunol Lett. 2012;147(1-2):29-33. doi:10.1016/j.imlet.2012.05.005
- Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106(48):20388-20393. doi:10.1073/pnas.0908698106
- Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999;5:28.
- Zhang H. Anti-IL-1β therapies. Recent Pat DNA Gene Seq. 2011;5(2):126-135. doi:10.2174/187221511796392024
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255-266. doi:10.1038/nri2056
- Apellis. Apellis announces 24-month results showing increased effects over time with pegcetacoplan in phase 3 DERBY and OAKS studies in geographic atrophy (GA). News release. August 24, 2022. Accessed December 20, 2022. https://investors.apellis.com/news-releases/news-release-details/apellis-announces-24-month-results-showing-increased-effects
- Aviceda Therapeutics. Aviceda announces successful completion of key IND toxicity milestone with favorable safety profile in non-human primates. Press release. December 5, 2022. Accessed January 4, 2023. https://www.businesswire.com/news/home/20221205005022/en/Aviceda-Announces-Successful-Completion-of-Key-IND-Toxicity-Milestone-with-Favorable-Safety-Profile-in-Non-Human-Primates
- Liu K, Wang L. Optogenetics: Therapeutic spark in neuropathic pain. Bosn J Basic Med Sci. 2019;19(4):321-327. doi:10.17305/bjbms.2019.4114
- GenSight Biologics. GS030 for geographic atrophy in dry-AMD. Accessed November 24, 2022. https://www.gensight-biologics.com/clinical-development-summary/#
- FOCUS: an open label first in human phase i/ii multicentre study to evaluate the safety, dose response and efficacy of gt005 administered as a single subretinal injection in subjects with macular atrophy due to AMD. Clinicaltrials.gov identifier: NCT03846193. Updated December 1, 2022. Accessed December 20, 2022. https://clinicaltrials.gov/ct2/show/NCT03846193
- Gyroscope Therapeutics announces positive interim data from phase 1 FOCUS trial of investigational gene therapy GT005. News release. Accessed December 20, 2022. https://www.gyroscopetx.com/gyroscope-therapeutics-announces-positive-interim-data-from-phase-i-ii-focus-trial-of-investigational-gene-therapy-gt005/
- Gyroscope. Clinical trials. Accessed December 20, 2022. https://www.gyroscopetx.com/clinical-trials/








