In 2023, the first FDA-approved drug for the treatment of geographic atrophy (GA) secondary to dry age-related macular degeneration (AMD) entered the market. Pegcetacoplan (Syfovre; Apellis Pharmaceuticals) is an inhibitor of complement C3. The approval of a complement inhibitor is a significant first step in GA treatment. However, despite the promising results of pegcetacoplan, there is still a limited number and variety of treatment options for patients with advanced dry AMD as well as room for improved efficacy and safety. Reductions in the rate of GA progression with complement inhibition have been promising in randomized clinical trials, but therapy may be associated with complications, such as increased rates of choroidal neovascularization (CNV), intraocular inflammation, and ischemic optic neuropathy.1 However, slowing the progression of GA may require more than complement inhibition alone, because there are other aspects of the inflammatory cascade involved in dry AMD that may require a multimodal approach.
Evidence is aligning around the theory that dry AMD is an inflammation-based disease.2 Chronic oxidative stress, abnormal complement activity, and chronic inflammation have been shown to contribute to AMD pathogenesis. Disruptions in retina homeostasis and insults to tissues may be critical factors in formative stages of the disease.
Resident mononuclear phagocytes like macrophages and microglia are vital to supporting retina homeostasis (Figure 1).3,4 Debris and waste from the highly metabolically active retina are cleared by phagocytes. Imbalances and insults incurred by the tissues trigger the recruitment of macrophages and microglia to the outer retina and Bruch’s membrane.4,5 Infiltrating macrophages react to local parainflammatory factors at the site of damage and switch to an M1 proinflammatory phenotype.3,4,6 An amplifying cycle results when proinflammatory factors released by these activated macrophages further recruit more inflammatory cells to the site of injury, perpetuating AMD progression. Current and emerging therapies have a role in the management of dry AMD, but by inhibiting complement factor alone, retina specialists are only able to target one aspect of the inflammatory process. Emerging clinical data suggest that there may be other key etiologic factors to examine apart from complement inhibition to target GA progression.

COMPLEMENT IS A VITAL PATHWAY
The complement cascade not only plays an important role in inflammation but also is a substantial component of the overall innate immune system. Complement signaling is vital to homeostasis, fighting infections, and promoting tissue remodeling/regeneration like synaptic pruning and remyelination.7 As a result, significant or complete blockade of complement has the potential for untoward downstream effects, such as increased risk of infection, central nervous system cell deterioration, and even angiogenesis.
Alternatively, regulation of complement pathway overactivation may dampen amplification loops seen in AMD disease progression. Both C3 and mononuclear phagocytes (macrophages and microglia) are necessary for axon regeneration.8 Basal levels of C3 and C5 independently display anti-angiogenic activity through macrophages.7 Deficiency of either of these complement factors increases neovascularization in the eye.7 In terms of future drug development, maintaining basal complement functions and crosstalk with immune cells may attenuate some of the complement depletion–associated adverse effects seen with the current treatment for GA.
COMPLEMENT WORKS WITHIN THE GREATER CELLULAR AND HUMORAL ARMS OF THE INNATE IMMUNE RESPONSE
The complement pathway fits into a larger picture of the innate immune response, which is comprised of 2 arms: the cellular arm and the humoral arm, the latter of which is where complement functions (Figure 2).9 Each arm contributes to the body’s overall immune response. In AMD, both can inflict tissue damage and contribute to disease pathogenesis. In the humoral arm, innate immune response is mediated by molecules like proinflammatory factors and complement activation.9 The cellular arm involves response of immune cells like macrophages and microglia.
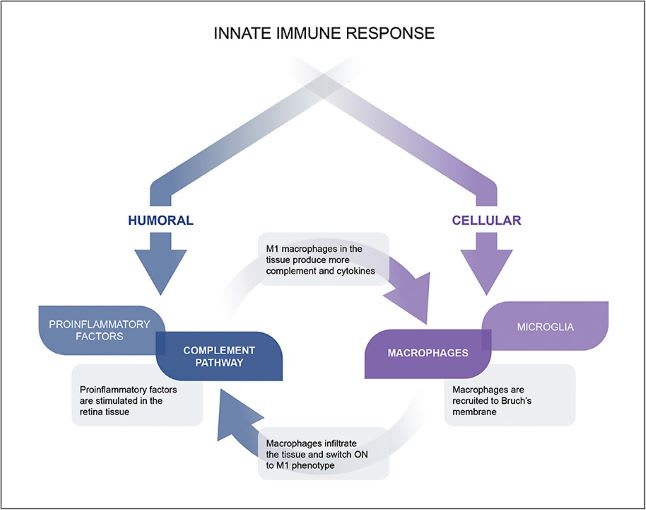
Evidence supports the formation of a positive feedback loop among the 2 independent arms of the immune system (Figure 2). In a positive feedback loop, it is no longer known if complement activation is the “chicken” or the “egg.” As such, inhibiting 1 arm of the immune response may not fully address its effect on AMD progression. Targeting both arms could provide broader global regulation of the innate immune response and dysfunction, halting feedback loop overamplification and reducing disease progression.
Retinal transcriptomic changes in early AMD disease stages recruit resident macrophages and microglia into the subretinal space.10,11 There, the infiltrating immune cells directly affect retinal pigment epithelium (RPE) function and viability.10,12 Additionally, key inflammatory factors are upregulated in the infiltrating cells.4 In AMD, macrophages are the primary producers of C3, and once they are recruited to Bruch’s membrane, they switch to the proinflammatory M1 phenotype. These interactions perpetuate the feedback loop and promote disease progression.6,11,13,14
Current GA treatments target 1 part of the immune system, usually a specific complement factor (eg, C3, C5, C1q). This does not educe the benefit of effectively regulating immune cell overactivation or approach the issue from multiple mechanisms. Thus, there is potential to explore the second arm of the immune response — the cellular arm — as a target for treatment of GA. A dual mechanism of action drug may target a global aspect of inflammation and innate immune dysfunction, rather than just targeting complement factors in the humoral arm. The potential benefit of this approach is global regulation of inflammation for potentially more effective treatment of GA secondary to AMD.
UNDEREXPLORED POTENTIAL IN THE CELLULAR ARM OF THE INNATE IMMUNE RESPONSE
Glycans (biologic sugar moieties) are expressed on the surface of all cells, and the moieties they form represent the glycome of the cell. Cells recognize other cells by unique glycome “barcodes,” allowing for communication, signaling, and identification.15,16 Unique moieties of bound glycans called sialic acids serve as structures for immune cells to identify other cells by. Importantly, immune cells can distinguish native “self” cells vs foreign “nonself” cells or distinguish between diseased and healthy tissue by recognizing these sialic acids.15,16
Glycoimmunology, or the role of glycans in immune response, is garnering large interest for drug development as a broader approach to resolving pathogenic inflammation. Immune cells express specific Siglec (sialic acid–binding immunoglobulin-type lectin) receptors that identify other cells by their sialic acids.16 If another cell is identified as “self” or homeostatic, immune response is shifted toward resolution so as not to attack the body.15,16 If a cell is identified as “nonself” or diseased, an innate immune response is mounted and macrophages activate to a proinflammatory M1 phenotype to attack the cell.15,16
Siglec recognition-response is the main inflammatory response mechanism for the innate immune system and macrophages. The key point of regulation in the interaction is the Siglec receptors that act as the on/off switch for the innate immune response and mononuclear phagocyte activation. Similar immune cell modulation is currently used in oncology with T-cell immune checkpoint inhibitors (ICI). Both Siglec modulation therapeutics and ICIs regulate immune activity through the same intracellular receptor signaling domains.17,18 Effective use of such immune regulation in oncology has inspired potential applications of Siglec receptor interactions in inflammatory retinal diseases like AMD.
Progress in glycoimmunology has bolstered evidence for the implication of Siglec receptors in AMD pathology. Retinal macrophages from AMD patient eyes have up to a 90-fold increase in Siglec receptor expression compared to nondiseased eyes.19 Siglec modulation has been shown to affect infiltrating macrophage polarization and activity in the retina.20-22 Taken together, cumulative data suggest that Siglecs can be effectively targeted to modulate infiltrating macrophages implicated in AMD pathology and have potential for therapeutic development in this disease.
TARGETING THE IMMUNE SYSTEM’S ON-OFF SWITCH TO POTENTIALLY TREAT GA
As described above, Siglec modulation can be leveraged as the on-off switch of the innate immune system. Both C3 and C5 are well-known factors in the proinflammatory complement pathway. Complement factor H (CFH) is produced locally and systemically by immune and RPE cells as a primary regulatory mechanism of the complement cascade.23 Complement factor H prevents overamplification of complement by inhibiting formation of C3bBb and C3 convertase. Genetic polymorphisms in the CFH gene have been strongly linked to the development of AMD.23-25
Complement factor H interacts with sialic acids independently of the binding that occurs between sialic acids and Siglec receptors on immune cells.26 While Siglec–sialic acid binding inhibits overactivated macrophages and promotes a proresolution phenotype, CFH–sialic acid binding further attenuates inflammation by inhibiting the complement cascade’s amplification/C3 activation step. Complement factor H dysfunction has been shown to promote AMD, and increasing CFH activity through sialic acid binding may favorably influence AMD pathology.
A DUAL MECHANISM OF ACTION TO POTENTIALLY TREAT GA
One promising glycoimmune therapeutic that acts through Siglec modulation is the investigational drug AVD-104 (Aviceda Therapeutics). AVD-104 is a sialic acid–coated nanoparticle administered via intravitreal injection that strongly binds to Siglec receptors on activated macrophages (Figure 3). Available data suggest that AVD-104 uses a dual mechanistic approach (Figure 4), regulating both arms (cellular and humoral) of the innate immune response. In the cellular arm, by binding to Siglecs on overactivated macrophages in the retina, AVD-104 induces the “self”/healthy tissue response in the macrophages, triggering repolarization to a resolution macrophage phenotype. Siglec receptors are also blocked from identifying abnormal sialic acids on diseased cells that would otherwise promote GA progression. This macrophage repolarization may also mitigate CNV conversion through phenotypic switches away from the pro-VEGF–producing, angiogenic M2 macrophage phenotypes to the resolution macrophage phenotype. Acting in the humoral arm, AVD-104–Siglec binding decreases complement factor overamplification through direct binding to CFH, which thereby downregulates C3 production in the immune cells.


The dual mechanism of action of AVD-104 both inhibits activated retina macrophages and halts overamplification of the complement pathway without complete depletion of C3 and C5 (Figure 4). Complete inhibition of C3 may promote adverse effects, such as intraocular inflammation and CNV conversion.7
In in vivo studies, AVD-104 decreased proinflammatory cytokines (IL-1ß, IL-12, IL-6, TNF-α), VEGF, and C3 levels as well as increased CFH activity in human macrophages.22,27 Biodistribution studies of the drug have shown activity in the RPE, sub-RPE, and choriocapillaris of rabbits 28 days after a single intravitreal injection. This translates into a potential dosing interval of 3 to 6 months in humans. By tackling the progression of advanced dry AMD with a dual mechanism of action on the immune system, AVD-104 may be effective in regulating immune overactivation in AMD from a comprehensive approach. Aviceda Therapeutics is actively enrolling and dosing participants in a multicenter phase 2/3 clinical trial for the treatment of GA secondary to dry AMD.
CONCLUSIONS
Significant advancements are being made in developing treatments for GA secondary to dry AMD. The introduction of complement inhibitor therapeutics has pioneered a rich pipeline for other modalities to treat dry AMD. The approval of pegcetacoplan has provided the first step in management of GA, but the frequent dosing interval, adverse events, and minimal functional benefit may suggest that there continues to be an unmet need to develop more effective and safer therapeutics. Complement plays a large role in the landscape of GA pathophysiology, but C3 and C5 inhibition have been shown to contribute to CNV and other adverse events, highlighting a need for other mechanistic approaches.
The prominent role of macrophages in AMD disease progression is garnering increased attention. Although they are implicated in GA and AMD, the exact sequence of events is not yet elucidated. Mounting evidence suggests that a positive feedback loop exists between immune cells and complement factors in the humoral arm of the innate immune response, with each part driving the other to amplify inflammation in retinal tissues.
Siglec receptors on activated macrophages appear to be a key point for regulation of this inflammatory response. Targeting this immune cell regulator, the on-off switch, may prove successful in altering the overactivated immune response in AMD. A similar mechanism has been widely implemented in the oncology space with immune checkpoint inhibitors.
Investigational AVD-104 is demonstrating encouraging in vivo results and is in phase 2/3 clinical trials. Available data suggest its dual mechanism of action may regulate both the humoral and cellular aspects of the immune response in AMD. By repolarizing activated M1/M2 macrophages to the resolution state and activating CFH to halt the overamplification of the complement system, AVD-104 has the potential to treat AMD from multiple angles with prolonged ocular durability. Glycoimmunology holds promise for the next innovation in the treatment of GA. Future results will guide development of glycoimmune therapies and potentially provide the ophthalmology community with additional tools and approaches to treat patients with AMD. RP
REFERENCES
- Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186-195. doi:10.1016/j.ophtha.2019.07.011
- Knickelbein JE, Chan CC, Sen HN, Ferris FL, Nussenblatt RB. Inflammatory mechanisms of age-related macular degeneration. Int Ophthalmol Clin. 2015;55(3):63-78. doi:10.1097/IIO.0000000000000073
- Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F. On phagocytes and macular degeneration. Prog Retin Eye Res. 2017;61:98-128. doi:10.1016/j.preteyeres.2017.06.002
- Wong JHC, Ma JYW, Jobling AI, et al. Exploring the pathogenesis of age-related macular degeneration: a review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front Neurosci. 2022;16. doi:10.3389/fnins.2022.1009599
- Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94(7):918-925. doi:10.1136/bjo.2009.165563
- Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61(9):528-535. doi:10.1111/j.1440-1827.2011.02695.x
- Langer HF, Chung KJ, Orlova VV, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116(22):4395-4403. doi:10.1182/blood-2010-01-261503
- Peterson SL, Li Y, Sun CJ, et al. Retinal ganglion cell axon regeneration requires complement and myeloid cell activity within the optic nerve. J Neurosci. 2021;41(41):8508-8531. doi:10.1523/JNEUROSCI.0555-21.2021
- Mantovani A, Garlanda C. Humoral innate immunity and acute-phase proteins. N Engl J Med. 2023;388(5):439-452. doi:10.1056/NEJMra2206346
- Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology. 2010;215(9-10):685-691. doi:10.1016/j.imbio.2010.05.010
- Lad EM, Cousins SW, Van Arnam JS, Proia AD. Abundance of infiltrating CD163+ cells in the retina of postmortem eyes with dry and neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1941-1945. doi:10.1007/s00417-015-3094-z
- Bonilha VL, Bell BA, Hu J, et al. Geographic atrophy: confocal scanning laser ophthalmoscopy, histology, and inflammation in the region of expanding lesions. Investig Opthalmology Vis Sci. 2020;61(8):15. doi:10.1167/iovs.61.8.15
- Cruz-Guilloty F, Saeed AM, Echegaray JJ, et al. Infiltration of proinflammatory M1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflamm. 2013;2013:e503725. doi:10.1155/2013/503725
- Natoli R, Fernando N, Jiao H, et al. Retinal macrophages synthesize C3 and activate complement in AMD and in models of focal retinal degeneration. Investig Opthalmology Vis Sci. 2017;58(7):2977. doi:10.1167/iovs.17-21672
- Gianchecchi E, Arena A, Fierabracci A. Sialic acid-siglec axis in human immune regulation, involvement in autoimmunity and cancer and potential therapeutic treatments. Int J Mol Sci. 2021;22(11):5774. doi:10.3390/ijms22115774
- Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30(1):357-392. doi:10.1146/annurev-immunol-020711-075018
- Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45. doi:10.1186/s13045-021-01056-8
- Gray MA, Stanczak MA, Mantuano NR, et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat Chem Biol. 2020;16(12):1376-1384. doi:10.1038/s41589-020-0622-x
- Patel D, Lad A, Callanan D, et al. Targeting self-recognition pattern receptors on retina immune cells with an engineered glycan-coated nanoparticle as a novel therapy for nonexudative AMD. Invest Ophthalmol Vis Sci. 2023;64(8):2959-2959.
- Shahraz A, Kopatz J, Mathy R, et al. Anti-inflammatory activity of low molecular weight polysialic acid on human macrophages. Sci Rep. 2015;5(1):16800. doi:10.1038/srep16800
- Karlstetter M, Kopatz J, Aslanidis A, et al. Polysialic acid blocks mononuclear phagocyte reactivity, inhibits complement activation, and protects from vascular damage in the retina. EMBO Mol Med. 2017;9(2):154-166. doi:10.15252/emmm.201606627
- Krishnan A, Patel D, Sendra VG, et al. Modulation of retinal inflammatory macrophages by sialic-acid coated nanoparticles as novel mechanism for nonexudative AMD treatment. Invest Ophthalmol Vis Sci. 2023;64(8):2730-2730.
- Parente R, Clark SJ, Inforzato A, Day AJ. Complement factor H in host defense and immune evasion. Cell Mol Life Sci. 2017;74(9):1605-1624. doi:10.1007/s00018-016-2418-4
- Landowski M, Kelly U, Klingeborn M, et al. Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. Proc Natl Acad Sci. 2019;116(9):3703-3711. doi:10.1073/pnas.1814014116
- Altay L, Sitnilska V, Schick T, et al. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye. 2019;33(12):1859-1864. doi:10.1038/s41433-019-0501-4
- Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T. Structural basis for sialic acid–mediated self-recognition by complement factor H. Nat Chem Biol. 2015;11(1):77-82. doi:10.1038/nchembio.1696
- Kuppermann BD, Callanan D, Hassan T, et al. Modulation of macrophages and complement dysfunction in nonexudative age-related macular degeneration utilizing a sialic-acid coated nanoparticle. Invest Ophthalmol Vis Sci. 2023;64(8):3239.








