Neurotrophic keratitis is characterized by decreased or absent sensation in the cornea. This is traditionally a result of injury to the nasociliary branch of the trigeminal (CN V) nerve, which provides sensation to the cornea. Neurotrophic keratitis can occur for several reasons, including diabetes, multiple sclerosis, herpes zoster, chemical burns, prolonged contact use, corneal or retinal surgeries, and any other trauma to or compression of the nasociliary nerve.1,2 Patients with neurotrophic keratitis have an altered blink reflex, decreased tear production, and poor corneal healing and regeneration that can result in persistent erosion of the cornea and scarring.2 As the disease progresses, patients can suffer permanent vision loss that affects their quality of life. Although this clinical entity is rare, affecting less than 5 people out of every 10,000, it also is often misdiagnosed and difficult to treat.3
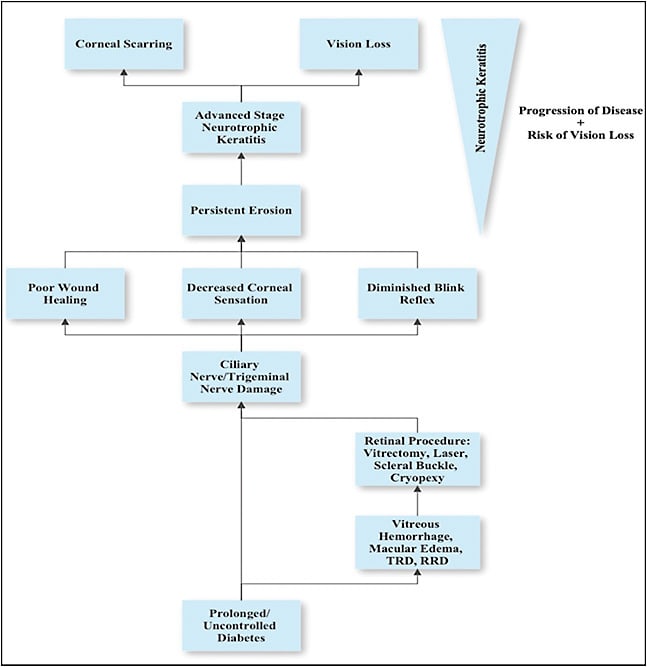
Diabetes has been identified as a risk factor for neurotrophic keratitis. Diabetes also leads to changes within the retina, such as proliferative diabetic retinopathy (PDR), retinal neovascularization, macular edema, vitreous hemorrhages, and retinal detachments.4 Many of these complications require invasive treatments that put the nasociliary branch of the trigeminal nerve at risk of trauma or iatrogenic damage and therefore increasing the risk of neurotrophic keratitis.5 As a result, diabetic patients may require careful monitoring of the cornea (in addition to the retina) following retinal procedures such as laser retinopexy, cryoretinopexy, and pars plana vitrectomy.
PATHOPHYSIOLOGY OF NEUROTROPHIC KERATITIS IN DIABETIC PATIENTS AFTER DIABETIC VITRECTOMY
Prolonged or uncontrolled diabetes results in neuropathies throughout the body, including the trigeminal nerve, which can lead to delayed corneal healing or regeneration, decreased corneal sensation, and an altered blinking reflex.2 Patients with PDR may require panretinal photocoagulation (PRP), which may lead to inadvertent damage to the long ciliary nerves. Patients with PDR may also develop nonclearing vitreous hemorrhages or tractional retinal detachments, necessitating more invasive management like vitrectomy or endolaser.4 Other diabetic patients may have vitreoretinal pathology not related to their diabetes, such as rhegmatogenous retinal detachments (RRDs), which may require vitrectomy, endolaser, and/or scleral buckling. Scleral buckling can also potentially impact the ciliary nerves by damage from indentation and/or cryopexy and has been associated with decreased corneal sensation.6,7 As a result, patients can develop persistent erosion of the cornea, therefore, leading to ulcers and ultimately causing vision loss.
HOW TO DIAGNOSE NEUROTROPHIC KERATITIS
Lacking corneal sensation, patients with neurotrophic keratitis do not often complain of many symptoms outside of blurred vision and potentially some conjunctival injection.8 However, the physical exam can often mimic dry eye disease with decreased tear break-up time and increased fluorescein staining.9 Neurotrophic keratitis can therefore often be misdiagnosed as dry eye disease.1 Physicians should become suspicious if the patient is failing to improve with typical dry eye treatment. For retina specialists, it may be beneficial to refer the patient to a cornea specialist for an evaluation of neurotrophic keratitis. Other conditions to consider in the differential diagnosis should include herpes simplex/herpes zoster, keratoconjunctivitis, medicamentosa keratoconjunctivitis, keratopathy related to topical nonsteroidal anti-inflammatory use, corneal limbal deficiency, and postoperative corneal melt.10 Multiple tools can be used to differentiate between a common ophthalmic disease like dry eye disease and a rare one such as neurotrophic keratitis. Patients suspected of having neurotrophic keratitis should undergo a full workup including careful examination of the eyelids and anterior segment along with an appropriate medical and surgical history which may help in making the diagnosis.11
Corneal sensitivity can be quantitatively assessed using techniques including Cochet-Bonnet or a noncontact gas esthesiometer, but assessment also can be done simply with a cotton swab in the office. The latter is more commonly performed and offers a qualitative method to assess corneal sensitivity. No topical anesthetic agent should be used before testing the patient. Approaching the eye from the side, the physician should gently touch the cornea with a wisped cotton swab in all 4 quadrants. The patient’s responses are compared between their 2 eyes, and the responses are categorized as normal, diminished, or absent. Patients with diminished or absent responses are potential eyes with neurotrophic keratitis.9,12 The cornea should also be assessed using fluorescein dye to look for any potential defects. The eyelids should be carefully examined for any defects followed by tear functioning tests (Schirmer tests and tear break-up time) and a patient’s blink rate should be assessed.9 Microbiologic tests can also be performed to rule out herpetic, fungal, bacterial, or acanthamoeba infections.3 Abnormal results, particularly for corneal sensitivity testing, are highly confirmative for neurotrophic keratitis. Once the disease is diagnosed, the patient should be appropriately staged and treated.
The Mackie classification system was developed to stage neurotrophic keratitis.13 Stage 1 (mild neurotrophic keratitis) is characterized by corneal abnormalities like epithelial hyperplasia and irregularity as well as Gaule spots (dry epithelium patches), punctate keratopathy, rose bengal or lissamine green staining of the inferior conjunctiva, increased viscosity of tear mucus, decreased tear break-up time, superficial neovascularization, stromal scarring, and/or dellen (elevation and thinning of the peripheral cornea at the limbus).8 Progression of the disease to stage 2 (moderate neurotrophic keratitis) includes persistent/worsening corneal epithelial defects and folds of Descemet’s membrane accompanied by stromal swelling, and possibly inflammation of the anterior chamber with hypopyon and or cells.8 Stage 3 (severe neurotrophic keratitis) represents the final stage of the disease and is characterized by corneal ulcers, perforation, and/or stromal melting.8 The risk of vision loss and corneal scarring increases with progression of the disease.8 Once the patient is staged, an appropriate management course can be initiated, which is often most effectively guided by a cornea specialist.
PREVENTION AND TREATMENT OF NEUROTROPHIC KERATITIS
Neurotrophic keratitis requires close monitoring and adaptations to the treatment regimen by the cornea specialist based on the success of interventions, and it is vital to quickly pivot to more invasive therapies if the disease is worsening. Conservative treatments are used initially, particularly for mild neurotrophic keratitis, with more invasive treatments being required as the disease progresses to stages 2 and 3. Patients diagnosed with stage 1 are frequently instructed to discontinue any drops that contain preservatives. Then preservative-free artificial tears are started and the patient can also receive treatment to try to control the symptoms of ocular surface disease with punctual plugs.1 These treatments have had varying levels of success, but many patients with more advanced disease (stages 2 and 3) may be especially difficult to treat.8 Stage 2 requires more aggressive treatment including the interventions outlined for stage 1, but also corneal and scleral contact lenses, amniotic membrane transplant, botulinum A toxin injections, and or surgical tarsorrhaphy.8

Stage 3 requires the most aggressive treatment to ensure no further damage or loss of vision results. Treatments for stage 3 have traditionally included bandage contact lenses, corneal gluing (cyanoacrylate) for perforation, conjunctival flap with tarsorrhaphy, and lamellar or penetrating keratoplasty (corneal transplant).1,8 Patients with stage 3 are at high risk of losing vision. Cenegermin-bkbj (Oxervate; Dompe), a recombinant form of human nerve growth factor, is a relatively new FDA-approved topical medication for neurotrophic keratitis. This treatment promotes corneal innervation, tear secretion, and epithelial cell growth.14 Clinical trial data showed that 70% of patients treated with Oxervate had complete corneal healing after 8 weeks.15 Unfortunately, the cost of the treatment (median out-of-pocket expense for Medicare patients was $5,791 in 2019-2020) is a potential barrier to the initiation of treatment in some patients.14 Finally, for diabetic patients, it is critical to ensure that their hemoglobin A1c is within normal limits to minimize additional damage to the trigeminal nerve and decrease the progression of diabetic corneal neuropathy.16 The complexity of diagnosing and treating neurotrophic keratitis often requires a multidisciplinary team to monitor the disease and prevent progression.
CONCLUSION
Diagnosing and treating neurotrophic keratitis is difficult for several reasons. For patients with neurotrophic keratitis, treatments are often focused on improving tear production and function, preventing inflammation and infection, repairing damaged cornea, and protecting the cornea from additional injury. Cenegermin-bkbj is a relatively new treatment specifically for neurotrophic keratitis that yields promising results.14,15 Diabetes is just one cause of neurotrophic keratitis, but patients and physicians would benefit from improved diagnostic efforts and monitoring after retinal procedures such as laser, vitrectomy, and scleral buckling surgeries. Retinal specialists could consider prophylactic treatment with preservative-free artificial tears in diabetic patients following retinal surgery or extensive PRP laser.1 Retinal physicians should have a low threshold for appropriate referrals to anterior-segment colleagues in patients with postoperative corneal changes, especially if the changes are not improving with typical dry eye treatments. Finally, ensuring that patients are being followed and treated by their primary care physician or endocrinologist for systemic control of their disease will limit the risk of damage to the trigeminal nerve and decrease the incidence of neurotrophic keratitis. RP
REFERENCES
- Israilevich RN, Syed ZA, Xu D, et al. Neurotrophic keratopathy following rhegmatogenous retinal detachment surgery [published online ahead of print, 2023 Jun 14]. Can J Ophthalmol. 2023;S0008-4182(23)00172-2. doi:10.1016/j.jcjo.2023.05.015
- Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37-45. doi:10.2147/EB.S117261
- Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves: the role of corneal nerves in neurotrophic keratitis. J Cell Physiol. 2017;232(4):717-724. doi:10.1002/jcp.25623
- Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015;6(3):489-499. doi:10.4239/wjd.v6.i3.489
- NaPier E, Camacho M, McDevitt TF, Sweeney AR. Neurotrophic keratopathy: current challenges and future prospects. Ann Med. 2022;54(1):666-673. doi:10.1080/07853890.2022.2045035
- Gibson RA. Reduction of corneal sensitivity after retinal detachment surgery. Br J Ophthalmol. 1981;65(9):614-617. doi:10.1136/bjo.65.9.614
- Dirani A, Antaki F, Rhéaume MA, et al. 360-degree intra-operative laser retinopexy for the prevention of retinal re-detachment in patients treated with primary pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2020;258(2):249-256. doi:10.1007/s00417-019-04534-5
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-579. doi:10.2147/OPTH.S45921
- Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107-131. doi:10.1016/j.preteyeres.2018.04.003
- Semeraro F, Forbice E, Romano V, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191-197. doi:10.1159/000354380
- Davis EA, Dohlman CH. Neurotrophic keratitis. Int Ophthalmol Clin. 2001;41(1).
- Jeng BH. 7 - diagnostic techniques in ocular surface disease. In: Holland EJ, Mannis MJ, Lee WB, eds. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. W.B. Saunders; 2013:47-54. doi:https://doi.org/10.1016/B978-1-4557-2876-3.00007-9
- Mackie I, Fraunfelder F, Roy F. Current Ocular Therapy. 4th ed. 1995.
- Dai X, Jensen A, Dun C, Karakus S, Rajaii F, Woreta F. Cost and prescriber and patient characteristics of cenegermin use in the Medicare population. Am J Ophthalmol. 2023;250:12-19. doi:10.1016/j.ajo.2023.01.025
- Sheha H, Tighe S, Hashem O, Hayashida Y. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973-1980. doi:10.2147/OPTH.S185184
- Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC. Diabetic corneal neuropathy. J Clin Med. 2020;9(12):3956. doi:10.3390/jcm9123956








