Infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), can lead to multiorgan system dysfunction1 and has killed more than 6.9 million people globally since December 2019.2 The eye is one of the organ systems affected by the sequelae of COVID-19. Ocular findings from COVID-19 infection vary, involving the anterior segment (conjunctivitis) to the posterior segment (retinal tissue and microvasculature) of the eye.3,4 It is known that the SARS-CoV-2 virus binds via protein S to the angiotensin-converting enzyme 2 (ACE2) receptor in the retina and choroid, which is also seen at higher rates in the heart, lungs, gastrointestinal tract, and kidney.5 The virus also damages the endothelial cells, leading to endothelial dysfunction and cytokine overproduction, causing vascular compromise such as hypercoagulability, ischemia, and edema.5,6
EFFECTS OF COVID-19 INFECTION IN THE RETINA
Neurologic manifestations have been reported in COVID-19 cases.7 Gross and microscopic changes have been reported affecting the brain.8,9 This resulted from an inflammatory process resulting in microglia activation. Because the retina is formed from the same components as the rest of the central nervous system, alteration of retinal structures is a concern. In addition, the retina is one of the most metabolically active tissues in the body, consuming oxygen more rapidly than most tissues.10 Because the virus enters the host cells via ACE2 receptor expressed in the vascular endothelium and neurosensory retina,11 retinal microvascular complications of COVID-19 would not be unexpected.
Several researchers have investigated anatomic and microvascular alterations and complications of SARS-CoV-2 in the retina. Others have also compared the retinal effects to the severity of COVID-19. One of the first reported retinal manifestations of COVID-19 infection observed funduscopic findings such as cotton-wool spots and microhemorrhages, as well as inner retinal hyperreflectivity using optical coherence tomography (OCT).12 Since then, series of cases reported vaso-occlusive events after infection.13-16 It is difficult to ascertain whether retinovascular findings are due to the COVID-19 virus itself or associated with the underlying conditions that many patients with more advanced COVID-19 infections have concurrently, such as hypertension and diabetes.
The retina has 2 unique oxygenation zones: the inner retina, supplied by autoregulated retinal vasculature, and the outer retina, supplied by nonautoregulated choroidal vasculature. Thus, the systemic oxygen level controls the oxygen level of the choroid.10 Despite the ability of the retinal vasculature to autoregulate, researchers noted a significant decrease in both the retinal and choroidal thickness measurements in patients who developed COVID-19 that developed pulmonary manifestations and hypoxia.17 Subsequent investigators explored using OCT angiography (OCTA), a noninvasive imaging that visualizes moving particles flowing through retinal vessels, to detect microvascular abnormalities. This imaging modality can help assess the presence of choroidal neovascularization and quantitatively assess the vascular density. Several groups concluded that patients infected with COVID-19 developed a decrease in the retinal vascular density with18 or without19 concomitant pneumonia, although in the former study,18 systemic vascular disease was included in their study cohort.
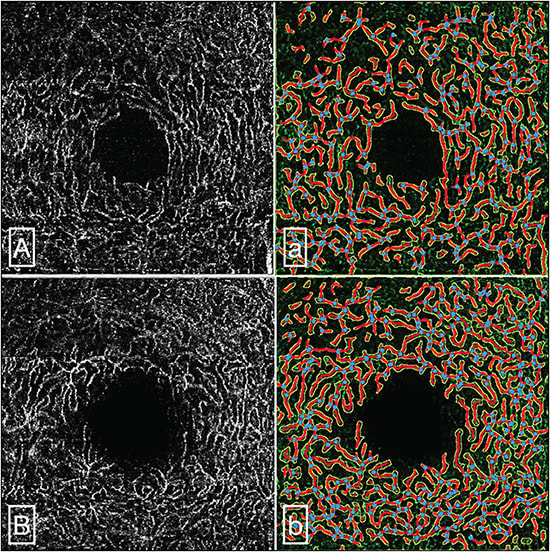
Retinal vascular density can also be influenced by vascular comorbidities like hypertension20 and diabetes,21 the most frequent comorbidities seen among patients with COVID-19, and these may be confounding factors to retinal changes seen in patients with COVID-19. In our prior work,22 we performed a cross-sectional study excluding these confounders and found that COVID severity, as measured by the requirement of hospitalization typically due to pneumonia, was associated with decreased retinal vascular density (Figure 1). This has been suggested by others in a systematic review as well.23,24 It is important to note, however, that for an OCTA to be usable and analyzable, proper segmentation correction of the different retinal layers should be observed to avoid false interpretation of flow signals from vascular slabs. Diseases that may promote disorganization of retinal anatomy may alter the proper segmentation of retinal layers. Hence, in cases where retinal tissue loss (eg, from COVID-19 infection) occurs, careful segmentation correction should be practiced.
Ocular inflammation has also been reported associated with COVID-19 infection.25 This includes uveitides and choroiditis such as multiple evanescent white dot syndrome,26 acute posterior multifocal placoid pigment epitheliopathy,27 anterior uveitis,28 and acute macular neuroretinopathy.29-32 The associations with certain uveitides were based on the temporal diagnosis of uveitis shortly after COVID-19 infection (range: same day up to 2 weeks). However, definitive evidence of COVID-19 causing uveitis is lacking.
EFFECTS OF COVID-19 VACCINATION IN THE RETINA
COVID vaccines have had an extremely positive impact on the risk and severity of infection. As of June 2023, more than 13 billion vaccine doses have been given worldwide.2 The common vaccine types are the following: mRNA, viral vector (adenovirus), inactivated virus, and protein subunits.33 Although potentially less effective, the inactivated virus vaccine has also been widely used. mRNA vaccines produce coronavirus proteins to generate a systemic immune response and there has been a suggestion of rare cases of uveitis (anterior, intermediate, and posterior) noted within 2 weeks of vaccination.34,35 Scleritis and episcleritis cases were also noted within 30 days from vaccination.36 It is difficult to determine cause and effect, and since hundreds of millions have been vaccinated, case reports of uveitis would be expected at a normal frequency despite exposure to vaccination. It is notable that most adverse effects from COVID-19 vaccination were transient and observed to resolve independently without vision-threatening sequelae.37 Compared to the microvascular consequences of COVID-19 infection, there is insufficient evidence of a causative change in retinal vessels due to vaccination. A group of researchers prospectively evaluated the density of retinal vasculature in health care workers. They evaluated the density with OCTA before a COVID-19 mRNA vaccine, after a COVID-19 mRNA vaccine, and 3 days after vaccination and noted a reduction in capillary density. They did not determine if the reduction was transient or permanent, or its effect on visual acuity,38 and thus this study must be considered inconclusive at the current time.
As acute COVID-19 complications become less common, long-term complications of prior COVID-19 disease have emerged. Patients experiencing new symptoms after the acute infection, termed post-acute sequela of SARS-CoV-2 infection (PASC) or long COVID, vary in incidence and prevalence widely. The mechanism is still incompletely understood, but it has been proposed to involve tissue and microvascular dysregulation.39 Future studies of long COVID patients and COVID patients who developed organ damage will allow insight into the vascular pathophysiology of COVID. OCTA may be a biomarker for complications of COVID-19 once future studies are completed. OCTA is a relatively quick and noninvasive procedure for acquiring retinal microvascular images from patients, and it may be beneficial to monitor PASC progression. Although visual disturbance can be transient, the long-term morphologic consequences of COVID-19 on retinal capillaries are unknown and further studies of macular vessels in COVID-19 should be considered.
CONCLUSION
Although reports from several studies demonstrated retinal microvascular and uveal involvement after either COVID-19 infection or vaccination, direct association and causation are still inconclusive, and these associations may be purely coincidental. Attention to the effects of PASC in the retina should be given importance because this is one of the emerging complications of COVID-19 given the large number of COVID-19 survivors and vaccination recipients. RP
REFERENCES
- Higgins CA, Nilsson-Payant BE, Bonaventure B, et al. SARS-CoV-2 hijacks p38β/MAPK11 to promote virus replication. mBio. 2023;e0100723. doi:10.1128/mbio.01007-23
- World Health Organization. WHO coronavirus (COVID-19) dashboard. Accessed September 11, 2023. https://covid19.who.int/
- Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye: a review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69(3):488-509. doi:10.4103/ijo.IJO_297_21
- Bertoli F, Veritti D, Danese C, et al. Ocular findings in COVID-19 patients: a review of direct manifestations and indirect effects on the eye. J Ophthalmol. 2020;2020:4827304. doi:10.1155/2020/4827304
- Kal M, Winiarczyk M, Zarębska-Michaluk D, et al. Long-term effect of SARS-CoV-2 infection on the retinal and choroidal microvasculature. J Clin Med. 2023;12(7):2528. doi:10.3390/jcm12072528
- Kazantzis D, Machairoudia G, Theodossiadis G, Theodossiadis P, Chatziralli I. Retinal microvascular changes in patients recovered from COVID-19 compared to healthy controls: a meta-analysis. Photodiagnosis Photodyn Ther. 2023;42:103556. doi:10.1016/j.pdpdt.2023.103556
- Abrishami M, Emamverdian Z, Shoeibi N, et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol. 2021;56(1):24-30. doi:10.1016/j.jcjo.2020.11.006
- Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
- Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697-707. doi:10.1038/s41586-022-04569-5
- Wangsa-Wirawan ND. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547. doi:10.1001/archopht.121.4.547
- Mavi Yildiz A, Ucan Gunduz G, Yalcinbayir O, Acet Ozturk NA, Avci R, Coskun F. SD-OCT assessment of macular and optic nerve alterations in patients recovered from COVID-19. Can J Ophthalmol. 2022;57(2):75-81. doi:10.1016/j.jcjo.2021.06.019
- Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. The Lancet. 2020;395(10237):1610. doi:10.1016/S0140-6736(20)31014-X
- Yalçınbayır Ö, Uçan Gündüz G, Coşkun F, Hakyemez B, Doğanay S. Different cases, different manifestations of post-COVID-19 retinal artery occlusion: a case series. Turk J Ophthalmol. 2023;53(2):124-129. doi:10.4274/tjo.galenos.2022.36930
- De Oliveira M, Lucena AV, Higino T, Ventura C. Central retinal artery occlusion with cilioretinal artery sparing secondary to COVID-19: additional ocular complication. Indian J Ophthalmol. 2023;71(2):663. doi:10.4103/ijo.IJO_1246_22
- Naughton A, Ong AY, Gkika T, Downes S. Bilateral paracentral acute middle maculopathy in a SARS-CoV-2-positive patient. Postgrad Med J. 2022;98(e2):e105-e106. doi:10.1136/postgradmedj-2021-140500
- Shiroma HF, Lima LH, Shiroma YB, et al. Retinal vascular occlusion in patients with the Covid-19 virus. Int J Retina Vitr. 2022;8(1):45. doi:10.1186/s40942-022-00371-7
- Güven YZ, Kıratlı K, Kahraman HG, Akay F, Yurdakul ES. Evaluation of acute effects of pulmonary involvement and hypoxia on retina and choroid in coronavirus disease 2019: an optic coherence tomography study. Photodiagnosis Photodyn Ther. 2023;41:103265. doi:10.1016/j.pdpdt.2022.103265
- D’Aloisio R, Ruggeri ML, D’Onofrio G, et al. Choroidal and retinal vascular findings in patients with COVID-19 complicated with pneumonia: widefield imaging. Diagnostics. 2023;13(6):1114. doi:10.3390/diagnostics13061114
- Urfalioğlu S, Akkök B, Özdemir G, Daghan B, Guler M. OCTA evaluation of posterior ocular blood flow in patients after COVID-19 infection without pneumonia. J Fr Ophtalmol. 2023;46(5):468-474. doi:10.1016/j.jfo.2023.01.002
- Lee WH, Park JH, Won Y, et al. Retinal microvascular change in hypertension as measured by optical coherence tomography angiography. Sci Rep. 2019;9(1):156. doi:10.1038/s41598-018-36474-1
- Di G, Weihong Y, Xiao Z, et al. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):873-879. doi:10.1007/s00417-015-3143-7
- Kalaw FGP, Warter A, Cavichini M, et al. Retinal tissue and microvasculature loss in COVID-19 infection. Sci Rep. 2023;13(1):5100. doi:10.1038/s41598-023-31835-x
- Teo KY, Invernizzi A, Staurenghi G, Cheung CMG. COVID-19-related retinal micro-vasculopathy – a review of current evidence. Am J Ophthalmol. 2022;235:98-110. doi:10.1016/j.ajo.2021.09.019
- Wang S, Wang J, Hu J, Wang N. Retinal microvascular impairment in COVID-19 patients: a meta-analysis. Immun Inflamm Dis. 2022;10(6). doi:10.1002/iid3.619
- Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99(8):1701-1707. doi:10.1007/s00277-020-04138-8
- Adzic Zecevic A, Vukovic D, Djurovic M, Lutovac Z, Zecevic K. Multiple evanescent white dot syndrome associated with coronavirus infection: a case report. Iran J Med Sci. 2023;48(1). doi:10.30476/ijms.2022.95007.2632
- Fischer NA, Wann RC, Crosson JN. Acute posterior multifocal placoid pigment epitheliopathy following COVID-19 infection. Am J Ophthalmol Case Rep. 2023;29:101790. doi:10.1016/j.ajoc.2022.101790
- Mohamadzadeh A, Mohamadzadeh D. Bilateral acute anterior uveitis and optic nerve edema as a manifestation of coronavirus disease-2019 (COVID-19): A case report. Clin Case Rep. 2023;11(6):e7473. doi:10.1002/ccr3.7473
- David JA, Fivgas GD. Acute macular neuroretinopathy associated with COVID-19 infection. Am J Ophthalmol Case Rep. 2021;24:101232. doi:10.1016/j.ajoc.2021.101232
- Dinh RH, Tsui E, Wieder MS, et al. Acute macular neuroretinopathy and coronavirus disease 2019. Ophthalmol Retina. 2023;7(2):198-200. doi:10.1016/j.oret.2022.09.005
- Giacuzzo C, Eandi CM, Kawasaki A. Bilateral acute macular neuroretinopathy following COVID-19 infection. Acta Ophthalmol (Copenh). 2022;100(2). doi:10.1111/aos.14913
- Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34(12):2352-2353. doi:10.1038/s41433-020-1069-8
- Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;14:3459-3476. doi:10.2147/IDR.S315727
- Testi I, Brandão-de-Resende C, De-La-Torre A, et al. Ocular inflammatory events following COVID-19 vaccination in the paediatric population: a multinational case series. Ocul Immunol Inflamm. 2023;1-6. doi:10.1080/09273948.2023.2220782
- Wang LU, Chen FT, Wang JK, et al. Ocular inflammatory manifestations following COVID-19 vaccinations in Taiwan: a case series. Taiwan J Ophthalmol. 2022;12(4):465. doi:10.4103/2211-5056.353129
- Sanjay S, Handa A, Kawali A, Shetty R, Bhakti Mishra S, Mahendradas P. Scleritis and episcleritis following coronavirus disease (COVID-19) vaccination. Ocul Immunol Inflamm. doi:10.1080/09273948.2023.2182324
- Kumari S, Anand R, Sambyal B, Singh Y, Rangappa P, Jha S. Ocular adverse effects of COVID-19 vaccines: a systematic review. J Fam Med Prim Care. 2022;11(9):5041. doi:10.4103/jfmpc.jfmpc_747_22
- Gedik B, Erol MK, Suren E, et al. Evaluation of retinal and optic disc vascular structures in individuals before and after Pfizer-BioNTech vaccination. Microvasc Res. 2023;147:104500. doi:10.1016/j.mvr.2023.104500
- Horwitz LI, Thaweethai T, Brosnahan SB, et al. Researching COVID to enhance recovery (RECOVER) adult study protocol: rationale, objectives, and design. PLoS One. 2023;18(6):e0286297. doi:10.1371/journal.pone.0286297








