The worldwide prevalence of diabetes was estimated to be 463 million in 2019 and is projected to continue to increase to 700 million patients by 2045.1 In 2021, the estimated number of patients living with diabetic retinopathy (DR) in the United States was 9.60 million people.2 Given the epidemic of diabetes, retina specialists and other eye care providers need to be aware of how to best manage this increasing number of patients. Despite the increasing prevalence of DR, the severity of the disease has diminished3 due to better screening protocols, earlier intervention, and improved treatment modalities. This article focuses on when to treat nonproliferative diabetic retinopathy (NPDR), what the options for treatment are, and interventions to prevent conversion to proliferative diabetic retinopathy (PDR).
WHEN TO TREAT DIABETIC MACULAR EDEMA
Historically, intravitreal anti-VEGF treatments for diabetic eye patients began with treating diabetic macular edema (DME). The phase 3 RISE/RIDE clinical trials demonstrated the importance of earlier treatment for patients with DME. A significantly greater proportion of patients in both the monthly ranibizumab (Lucentis; Genentech) 0.3 mg and 0.5 mg intravitreal injection groups compared to sham experienced visual acuity gains of ≥15 letters.4 Patients in the sham group who were crossed over to the treatment arm after 2 years did not attain similar visual acuity gains compared to those who were initiated early, suggesting that irreversible structural changes occur if DME is left untreated. However, how aggressive should we be when treating DME? The DRCR Retina Network’s Protocol V guides retina specialists to monitor patients with DME if their visual acuity is 20/25 or better.5 There was a nonsignificant difference in terms of the proportion of patients who lost >5 letters between the aflibercept (Eylea; Regeneron), grid/focal laser, and observation arms after 2 years.5 Given that clinical trials like RISE/RIDE and VIVID/VISTA excluded patients with vision better than 20/40, before Protocol V the retina community knew little about how to manage patients with DME and good vision. However, it is important to note that secondary post hoc analysis of Protocol V demonstrated that patients with a central subfoveal thickness of ≥300 μm, at least moderately severe NPDR or worse (Diabetic Retinopathy Severity Scale [DRSS] score >47), and fellow eyes that had received treatment for DME within 4 months of randomization were approximately twice as likely to require aflibercept therapy for visual acuity loss.6
Other important prognosticators for how aggressive retina specialists should be when treating DME are baseline visual acuity and central subfoveal thickness. The RESTORE study demonstrated that patients with best corrected visual acuity (BCVA) ≤60 ETDRS letters and those with >400 μm central retinal thickness had the greatest visual gains when ranibiziumab (or ranibizumab + laser) was initiated.7 Another clinical question that arises is how often retina specialists should inject patients. Per VIVID/VISTA, the impact on vision gains for every-4-week (q4) intravitreal aflibercept 2 mg dosing vs every-8-week (q8) dosing after an initial 5 loading doses was not significant.8 However, the proportion of patients who experienced a 3-step or more improvement in DRSS score was greater in both the aflibercept q4 week and aflibercept q8 week arms compared to the laser group; in addition to a smaller proportion of patients going on to develop PDR in both aflibercept groups as well.9
Newer agents such as faricimab (Vabysmo; Genentech/Roche) are also approved to treat DME, with many but not all patients having nearly equivalent gains in vision with 12-16 week dosing intervals. Modern trial designs such as YOSEMITE/RHINE (faricimab for DME) essentially try to replicate a treat-and-extend treatment paradigm in the major randomized clinical trial setting. It is also important to note that patients with NPDR and “mild” DME (eg, CST <325 μm) may not be included in clinical trials. In real-world practice, some retinal physicians may inject patients with mild disease to prevent them from going on to become worse. However, trial data to date do not fully capture the benefit of this paradigm.
OPTIONS TO TREAT
Focal and grid laser therapy for the treatment of DME has largely been supplanted by anti-VEGF therapy due to superior visual outcomes and often vision gains rather than prevention of vision loss. Studies have demonstrated the superior efficacy of anti-VEGF compared to laser in terms of visual gains.10,11 However, the type of therapy should be tailored to the individual patient. For example, patients who have multiple doctors’ appointments, significant comorbidities that may result in frequent hospitalization, or barriers to maintaining routine follow-up may not be able to be seen on a monthly basis for their intravitreal injections. Therefore, for patients with significant central or parafoveal exudation or for those where monthly intravitreal injections may not be realistic, fluorescein angiography can be helpful to find leaking microaneurysms and to guide focal laser therapy, particularly if they are not subfoveal and if it is a single leaking microaneurysm, for instance in the temporal macula. Nevertheless, anti-VEGF injections for DME remain the gold standard for the vast majority of cases in 2023.
Although primary anti-VEGF intravitreal therapy allows tremendous vision gains, there remain nonresponders to anti-VEGF monotherapy, and other inflammatory biomarkers and signaling cascades create a multifactorial milieu beyond VEGF. In January of 2022, the Food and Drug Administration approved faricimab (Vabysmo; Genentech), the first bispecific monoclonal antibody that simultaneously targets 2 key pathways: VEGF and angiopoietin-2 (Ang2).12,13 By inhibiting VEGF-A, there is less endothelial cell proliferation, reduced vascular permeability, and diminished drive for neovascularization.14 In addition, Ang2 is primarily produced by vascular endothelial cells and functions as a vessel destabilizing agent through its inhibition of Tie2 and direction competition with Ang1.15 Ang2 inhibition can therefore result in greater vessel stability and less susceptibility to the effects of VEGF-A. The YOSEMITE and RHINE clinical trials demonstrated noninferiority of intravitreal faricimab compared to aflibercept every 8 weeks, as well as the ability to extend patients up to 16 weeks.16 At the end of year 2, up to 60% of patients in the treat-and-extend arm were extended to 16 weeks, and 70% of patients were at q12 week dosing after 4 initial loading doses.16 It is possible that patients with persistent DME may benefit from switching to faricimab (Figure 1).
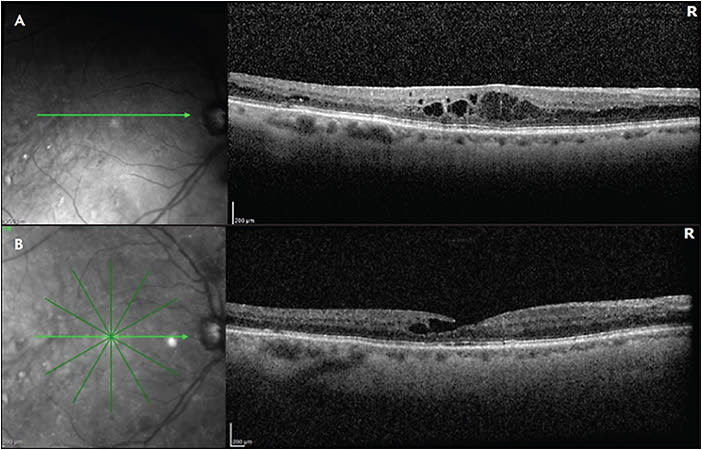
We know that anti-VEGF is not the only pathway that can serve as a therapeutic target in patients with DR. We learned from the DRCR Retina Network Protocol T that more than 30% of patients can still have persistent DME despite 4 intravitreal injections within 24 weeks.17 Inflammatory mediators such as interleukin-6, interleukin-8, monocyte chemoattractant-1, and pigment epithelium-derived factor may play a role in the pathogenesis of DME, in addition to VEGF.18 Therefore, steroids (intravitreal and possibly periocular) can serve as an important addition to our armamentarium to treat DME.
Protocol U evaluated the impact of addition of intravitreal dexamethasone 0.7 mg implant (Ozurdex; Allergan) to anti-VEGF therapy vs anti-VEGF alone in the treatment of DME. The study included 129 patients, all of whom had received at least 3 anti-VEGF injections within 20 weeks of enrollment and had persistent DME with visual acuity of 20/32 or worse. All patients received a loading dose of 3 ranibizumab injections and if they still met inclusion criteria, they were randomized to either combination ranibizumab and dexamethasone implant vs ranibizumab alone. After 6 months, there was not a statistically significant difference in average visual acuity gains between the combination group versus ranibizumab only — 3.0 letters vs 2.7 letters, respectively — however, there was an average of 110 μm reduction in central subfield thickness in the combination group compared to 62 μm reduction in the ranibizumab-only group.19 One could argue that if the trial had run for a longer period of time, there would have been a clinically and statistically significant difference in visual acuity gains.
On the contrary, in the MEAD study, 22% of patients in the dexamethasone 0.7 mg implant group achieved a ≥15 letter improvement in visual acuity, compared to 18% in the 0.35 mg group, and 12% in the sham group.20 The FAME study compared intravitreal fluocinolone acetonide 0.2 µg, 0.5 µg, and sham in the treatment of DME. The percentage of patients with a gain of 15 letters or more from baseline at month 24 was 28.7% and 28.6% in the low-dose and high-dose FA insert groups, respectively, compared with 16.2% in the sham group.21 The safety and efficacy profile favored the low-dose treatment arm, given the lower rate of cataract formation and less need for incisional glaucoma surgery.21
RISK OF PROGRESSION TO WORSE DISEASE
Retina specialists are always concerned about patients with nonproliferative disease going on to have worse disease, whether this is the development of DME or PDR, and the associated complications. Patients with risk factors such as uncontrolled systemic A1c or conversion to PDR in the fellow eye are of particular concern. The prespecified secondary outcomes in the DME trials of RISE/RIDE noted a significant decrease in DRSS scores in patients who were treated with intravitreal anti-VEGF but not with sham, leading to approval of intravitreal ranibizumab for DR (and not requiring DME for treatment). Studies like PANORAMA demonstrated that patients with moderate-to-severe NPDR with or without DME treated with aflibercept had significantly greater improvement in the DRSS score compared with sham.22 Moreover, there was a reduced risk of vision-threatening complications, such as center-involving DME (CI-DME) and conversion to PDR. This raises the question of whether retina specialists should initiate earlier treatment of patients with severe NPDR with anti-VEGF, regardless of DME status. DRCR Retina Network Protocol W sought to answer this question. This study included eyes with moderate-to-severe NPDR without baseline CI-DME and randomized patients to either aflibercept injection or sham. The study found that patients in the aflibercept group were nearly threefold less likely to develop CI-DME with associated vision loss compared to sham, and over twofold less likely to develop PDR at the 2-year mark.23 Nonetheless, visual acuity was not found to be significantly different between the 2 groups.23 It will be interesting to see what the 5-year results demonstrate given that chronic fluid and the duration of PDR negatively correlate with visual acuity.
One point that is not always considered is that although the visual acuity outcomes were no different in Protocol W if treatment was withheld until conversion to PDR, the patient experience and journey if an acute onset of neovascular glaucoma or anterior segment rubeosis occurs with need for urgent treatment is not accurately depicted by focusing on visual acuity results only. Therefore, an individualized treatment approach is needed for NPDR; anti-VEGF therapy for NPDR without DME is not only an FDA-approved option but also a helpful option for the right patient. For physicians who do not typically offer treatment of NPDR without DME, one scenario that can help introduce this treatment option is to observe DRSS score regression in a patient with unilateral DME that has been treated and consider initiating treatment in the fellow eye with NPDR without DME. This could be a helpful scenario for physicians who are contemplating introducing anti-VEGF to treat NPDR into their practice.
FUTURE DIRECTIONS
Treatment of diabetic eye disease has taken tremendous strides from an initial focus on treatment for DM to an expanded indication of DR without DME. With the array of injectable medications available, the next step will be looking at durability and efficacy to alleviate some of the treatment burden patients have with frequent visits and injections. Just recently, results from the PHOTON study demonstrated the efficacy of high-dose aflibercept (8 mg) for the treatment of DME. The study found that 89% of patients in the high-dose group maintained at least q12 week dosing through 2 years, and 83% maintained at least q16 week dosing.24 Based on these findings, the Food and Drug Administration approved high-dose aflibercept (Eylea HD; Regeneron) for the treatment of DME (and wet age-related macular degeneration) on August 18, 2023.
Aside from injectable medications, oral medications for the treatment of diabetic retinopathy and DME have made waves in the retnina community. For example, Ocuphire Pharma’s APX3330, a twice-daily oral tablet, was launched in 2021 and showed promising results in phase 1 and 2 clinical trials. It is a small molecule that targets apurinic/apyrimidinic endonuclease 1/redox effector factor-1 (Ref-1), and has been found to reduce abnormal angiogenesis and inflammation in preclinical models.25 Additionally, Occurx’s OCX-063 is an oral antifibrotic agent being studied in a number of ophthalmic conditions including proliferative vitreoretinopathy, neovascular age-related macular degeneration, and NPDR.26
Studies such as Protocol W continue to investigate the pros and cons of intravitreal anti-VEGF treatment for NPDR. However, there has been a focus on the fact that there is no difference in final visual acuity and this may lead to a “watch and wait” approach until development of PDR. However, this type of approach may not capture a patient’s experience and journey once they have converted to PDR.
CONCLUSION
Although a majority of retinal physicians may state they do not treat NPDR without DME, in 2023 we have increasing evidence and data to strongly consider anti-VEGF as a treatment option for appropriate cases, and encourage this discussion with patients with all the risks, benefits, and alternatives. Early detection and treatment of NDPR, specifically DME, remains an important role for the retina specialist in preventing vision loss in patients with diabetes. RP
REFERENCES
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
- Lundeen EA, Burke-Conte Z, Rein DB, et al. Prevalence of diabetic retinopathy in the US in 2021. JAMA Ophthalmol. Published online June 15, 2023. doi:10.1001/jamaophthalmol.2023.2289
- LeCaire TJ, Palta M, Klein R, Klein BEK, Cruickshanks KJ. Assessing progress in retinopathy outcomes in type 1 diabetes: comparing findings from the Wisconsin Diabetes Registry Study and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 2013;36(3):631-637. doi:10.2337/dc12-0863
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi:10.1016/j.ophtha.2011.12.039
- Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894. doi:10.1001/jama.2019.5790
- Glassman AR, Baker CW, Beaulieu WT, et al. Assessment of the DRCR Retina Network approach to management with initial observation for eyes with center-involved diabetic macular edema and good visual acuity: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2020;138(4):341-349. doi:10.1001/jamaophthalmol.2019.6035
- Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615-625. doi:10.1016/j.ophtha.2011.01.031
- Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044-2052. doi:10.1016/j.ophtha.2015.06.017
- Mitchell P, McAllister I, Larsen M, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retina. 2018;2(10):988-996. doi:10.1016/j.oret.2018.02.011
- Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. doi:10.1016/j.ophtha.2010.02.031
- Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381. doi:10.1016/j.ophtha.2014.08.047
- Peach CJ, Mignone VW, Arruda MA, et al. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Int J Mol Sci. 2018;19(4). doi:10.3390/ijms19041264
- Nicolò M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30(3):193-200. doi:10.1080/13543784.2021.1879791
- Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8(11):1265-1288. doi:10.15252/emmm.201505889
- Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235-239. doi:10.1038/nm1351
- Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet Lond Engl. 2022;399(10326):741-755. doi:10.1016/S0140-6736(22)00018-6
- Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257-269. doi:10.1001/jamaophthalmol.2017.6565
- Yoshimura T, Sonoda K hei, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PloS One. 2009;4(12):e8158. doi:10.1371/journal.pone.0008158
- Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR Network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136(1):29-38. doi:10.1001/jamaophthalmol.2017.4914
- Boyer DS, Yoon YH, Belfort RJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914. doi:10.1016/j.ophtha.2014.04.024
- Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e2. doi:10.1016/j.ophtha.2010.12.028
- Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946-955. doi:10.1001/jamaophthalmol.2021.2809
- Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139(7):701-712. doi:10.1001/jamaophthalmol.2021.0606
- Do DV. Aflibercept 8 mg for diabetic macular edema: 48-week results from the phase 2/3 PHOTON trial. Invest Ophthalmol Vis Sci. 2023;64(8):2814-2814.
- Silva LL, Lambert-Cheatham N, Stratford RE, et al. Oral APX3330 treatment reduces L-CNV lesions in preclinical mouse model and confirms phase 2 DR/DME clinical dose with sufficient distribution to human retina using PBPK modeling. Invest Ophthalmol Vis Sci. 2021;62(8):1073-1073.
- A phase I, randomised, double blind, placebo-controlled, dose-escalating study of the safety, tolerability, food effect and pharmacokinetics of single and repeat doses of OCX063 administered orally to healthy volunteers. Australian Clinical Trials. Accessed September 5, 2023. https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12619001607167








