This article was originally published in a sponsored newsletter.
The approvals of pegcetacoplan (Syfovre; Apellis) and avacincaptad pegol (Izervay; Iveric Bio) have ushered in a new era for the treatment of geographic atrophy (GA), a devastating disease that previously had no therapeutic options. Now more than ever, it is imperative to diagnose patients with GA early, identify those at high risk of progression, and determine who would benefit most from these novel treatments. Fortunately, retina specialists today have several tools in their arsenal to assist with this task, including color fundus photography, optical coherence tomography (OCT), fundus autofluorescence (FAF), fluorescein angiography, and confocal scanning laser ophthalmoscopy. Here, we will focus on the most commonly used modalities and the imaging biomarkers that can be used to predict the development and progression of GA.
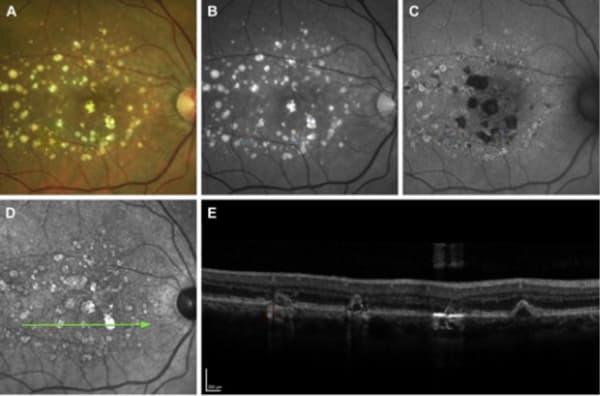
FAF is one of the most common methods for identifying and monitoring GA, and was used to assess the primary endpoint in the clinical trials for both FDA-approved drugs. FAF captures fluorescent signals emitted by lipofuscin in retinal pigment epithelium (RPE) cells. GA appears as dark or hypoautofluorescent patches on FAF due to the loss of RPE cells, and the FAF pattern can be classified as focal, banded, patchy, or diffuse. Banded or diffuse patterns are associated with a faster rate of GA progression, particularly the diffuse-trickling type. Other risk factors associated with GA progression include baseline lesion size, multifocality, non-foveal location, and focal rim hyperautofluorescence.
However, the most common and most useful imaging modality in GA is OCT because it provides high-resolution cross-sectional images of the retina and demonstrates several biomarkers that predict disease progression. In the early stages of AMD, OCT can be used to assess the presence, size, and distribution of drusen, which can help predict the long-term risk of developing GA independent of clinical factors such as age, smoking, or genetic susceptibility. Drusen that are larger or closer to the fovea are more likely to progress to GA. Similarly, drusen that are more refractile on OCT are also more likely to progress to GA. OCT can also identify other features including intraretinal hyper-reflective foci (the presence and concentration of which have been associated with the incidence and increase in GA lesions), hyperreflective crystalline deposits, and subretinal drusenoid deposits (previously called reticular pseudodrusen – a distinct entity that is correlated with advanced AMD and is often not well-visualized on biomicroscopy or fundus photography). In late stages, OCT can visualize GA as hypertransmission defects with RPE and outer retinal atrophy.
As we continue to learn more about the disease process, and as our imaging and diagnostic tools evolve, we will undoubtedly uncover more ways to identify GA at an early stage and prevent permanent vision loss for millions of patients.