Coats disease is a retinal vascular disorder characterized by aneurysmal and telangiectatic retinal vessels along with intraretinal or subretinal exudation and fluid. Scottish ophthalmologist George Coats, MD, first described the disease as a “very peculiar form of vascular disease” accompanied by an “extensive mass of exudation” in areas of the fundus.1 Coats developed an initial classification system of 3 groups based on the presence and severity of subretinal exudation, intraretinal hemorrhage, and arteriovenous (AV) malformations. However, this system was abandoned after certain components of the stages were identified as separate diseases altogether, such as retinal capillary hemangioma or retinal hemangioblastoma.1
Often synonymous with an early stage of Coats disease is Leber multiple miliary aneurysms. Leber multiple miliary aneurysms was first characterized in 1912 as aneurysmal and telangiectatic retinal vasculature without significant subretinal exudation.2 Although initially believed to be distinct from Coats disease, it is now understood to be an earlier stage of disease progression in Coats disease.
CLASSIFICATION
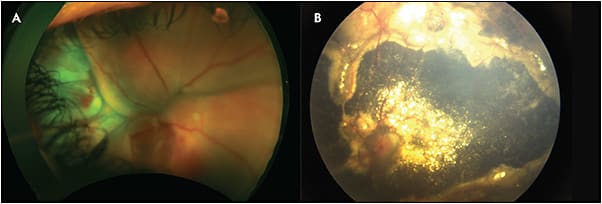
Although there were various staging systems for Coats disease, the classifications developed by Shields et al in 2000 are most commonly used and are broken up into 5 stages (Table 1).3 Stage 1 is characterized by retinal telangiectasias alone. Stage 2 is characterized by telangiectasias and exudation, with extrafoveal exudation being classified as stage 2A (Figure 1) and foveal exudation as stage 2B. Stage 3 is defined by the presence of exudative retinal detachment. Stage 3A (Figure 2) has subtotal detachment whereas stage 3B has total detachment. Stage 4 (Figure 3) has total retinal detachment along with increased intraocular pressure, and stage 5 is advanced end-stage diseases along with occasional phthisis bulbi, caused by secondary complications like neovascular glaucoma. Of the classification categories, stage 3 has the highest prevalence (68%), followed by stage 4 (15%) and stage 2 (14%). Stage 1 has the lowest prevalence of 1%.3 These rates have not changed significantly in the past 45 years despite an increase in pediatric ophthalmic screening.4
| STAGING OF COATS DISEASE | |
| Stage 1 | Retinal telangiectasias alone |
| Stage 2A | Telangiectasias with extrafoveal exudation |
| Stage 2B | Telangiectasias with subfoveal exudation |
| Stage 3A | Exudative subtotal retinal detachment; subgroups 3A1 extrafoveal and 3A2 foveal |
| Stage 3B | Exudative total retinal detachment |
| Stage 4 | Total retinal detachment and increased intraocular pressure |
| Stage 5 | Advanced end-stage diseases |
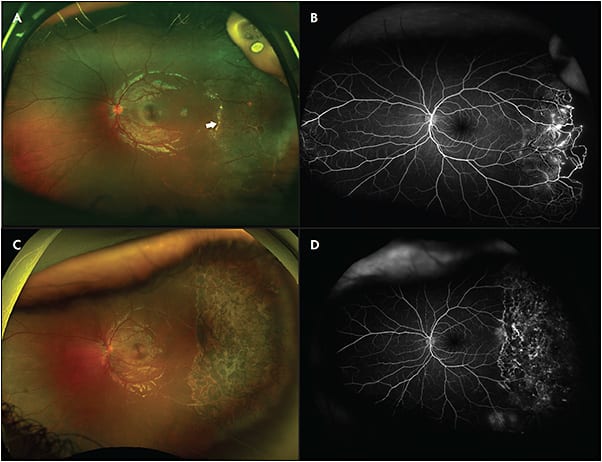
![Figure 2. Stage 3A1 Coats disease. A patient presented with an inferotemporal subtotal exudative retinal detachment with dense posterior exudation (white arrow on optical coherence tomography [OCT]) (A, B). After treatment with laser photocoagulation, intravitreal bevacizumab, and posterior sub-Tenon triamcinolone, the exudative detachment improved, but unfortunately the lipid exudation formed a subfoveal nodule (white arrow on OCT) (C, D). Images courtesy of Cynthia Toth, MD](https://res.cloudinary.com/broadcastmed/image/fetch/q_auto,f_auto/dpr_auto/https://retinalphysician.com/media/4wikkgh2/rp_nov_1801.jpg)
CLINICAL FEATURES AND SYMPTOMS
Coats disease is generally a painless, slowly progressive condition that is largely unilateral and occurs mostly in young, otherwise healthy males. The average age of onset is 5 years, but it has been diagnosed in patients ranging from 1 month to 63 years of age. Common symptoms associated with Coats disease include reduced visual acuity, strabismus, and xanthocoria.2 Early retinal changes involve the peripheral and equatorial retina more often than the posterior pole.
Common retinal findings include telangiectasias, intraretinal exudation, exudative retinal detachment, retinal hemorrhage, and retinal microcysts. The retinal vessels affected by Coats have been described as enveloped by yellow cholesterol deposits. They also have an irregular caliber with aneurysmal dilation, giving them the description of a “lightbulb.” On ophthalmoscopy, a common Coats disease presentation contains localized, yellow, subretinal exudation with adjacent vascular abnormalities such as sheathing, tortuosity, telangiectasia, and dilation.5 The anterior segment is generally normal in most patients, but abnormalities include presence of cataracts and corneal edema.2 Although there are various common presentations for Coats disease, 25% of patients can present without symptoms and are only diagnosed on routine eye exams.5
Coats disease manifestation in adults generally have similar presentations and clinical courses, but there is usually slower progression of disease, smaller area of involvement, and more frequent hemorrhage surrounding dilated vessels.2 Additionally, recent studies have demonstrated that the fellow eye could demonstrate abnormal findings including telangiectasias, aneurysms, nonperfusion, or leakage.6
DIFFERENTIAL DIAGNOSES
Given that the presentation of Coats disease can include common symptoms such as xanthocoria, strabismus, and reduced visual acuity, distinguishing Coats from other diseases can be challenging. Diseases with similar presenting symptoms include retinoblastoma, retinal detachment, persistent fetal vasculature, congenital cataract, Norrie disease, incontinentia pigmenti, and familial exudative vitreoretinopathy (FEVR).2 Shields et al found that among 150 cases of Coats disease, only 41% of cases were correctly diagnosed, whereas remaining cases were misclassified as retinoblastoma, retinal detachment, retinal hemorrhage, toxocariasis, choroidal melanoma, choroidal hemangioma, coloboma, endophthalmitis, cytomegalovirus retinitis, or no diagnosis.7 An especially important distinction to make is whether a patient presents with Coats disease or retinoblastoma given the similar presentation of xanthocoria (Table 2). If a patient presents with bilateral symptoms, the differential diagnosis includes retinoblastoma, FEVR, and Norrie disease.8
| COATS DISEASE | RETINOBLASTOMA | |
|---|---|---|
| Age of onset | 5 years | 18 months |
| Sex-based prevalence | More common in males | Similar prevalence between sexes |
| Unilateral prevalence | 95% to 100%26 | 60%27 |
| Ophthalmoscopy imaging findings | Yellow exudation without feeder vessels | White tumor with dilated feeder artery |
| Presence of exudate | Yes | No |
| Presence of retinal detachment | Yes | Yes |
| Subretinal detachment calcifications on b-scan | No | Yes |
| Computed tomography findings | Distinct retinal detachment, uniform opacification of vitreous cavity, and subretinal densities5 | High density, calcified mass with moderate enhancement after iodinated contrast usage28 |
| Magnetic resonance imaging findings | Subretinal space hyperintensity on T1, and subretinal space hyperintensity/hypointensity on T25 | Hyperintense mass relative to vitreous on T1 imaging and hypointense tumor on T2 with minimal hypointense foci within mass indicating calcifications29 |
DIAGNOSTIC STUDIES
The most commonly used and critical diagnostic tool for Coats disease is fluorescein angiography (FA), which can illustrate findings of telangiectasias, beading of vessel walls, and aneurysms (Figure 1).2 Fluorescein angiography can also identify leakage of vessels as a source of hemorrhage and exudation and identify treatable areas of nonperfusion. Ultrasonography can also be particularly beneficial when trying to distinguish Coats disease from other ophthalmologic diseases that cause xanthocoria, especially ocular tumors. Ultrasonography of Coats disease can show poorly mobile retinal detachments, subretinal cholesterol opacities, and absence of mass lesion. Computed tomography and magnetic resonance imaging can be considered in select circumstances in consultation with an ocular oncologist for cases suspicious for retinoblastoma (Table 2).
Optical coherence tomography (OCT) is useful for identifying subretinal fluid, fibrosis, exudates, and the presence or absence of foveal involvement. Outer retinal atrophy of the outer nuclear layer, retinal pigment epithelium, and ellipsoid zone may also be visualized on OCT in patients with extensive subretinal exudation.9,10 When compared to fundus photography and FA, OCT was able to identify exudates in multiple retinal layers, small pockets of subretinal fluid, outer retinal atrophy overlying fibrotic nodules, and small preretinal hyperreflective OCT dots that were not seen in other imaging modalities.11,12
MANAGEMENT
Treating Coats disease early in its progression is important due to the severe complications that can arise from untreated disease. One study found that of 22 patients with Coats disease who went untreated for 5 years, 14 developed total retinal detachment, and 7 developed secondary glaucoma. They also found that more severe and earlier age of presentation of disease were correlated with a more rapid decline in vision.13 The staging of Coats disease significantly impacts treatment options, such that stage 1 can be monitored with fundus photography, FA, and OCT. However, stage 2 or worse warrants treatment. The goal is to treat areas of nonperfusion and abnormal vasculature to prevent progression.
Less severe cases can be managed with argon or diode laser photocoagulation with guidance from FA imaging. Wavelengths in the yellow spectrum are particularly effective because they are better absorbed by blood in target vessels.2,14 Photocoagulation therapy often occurs over multiple sessions to effectively cover areas of abnormal vasculature and nonperfusion. In the authors’ experience, long duration (800 ms to 1,000 ms) treatment to abnormal vessels and regular duration (200 ms) scatter laser to areas of nonperfusion is an effective combination (Figure 1). Photocoagulation reactions could include inflammation and choroidal detachment. Finally, an initial progressive exudation after treatment prior to improvement is often noted (Figure 2).
In more advance cases of Coats disease, particularly with exudation or retinal or subretinal fluid, treatment can be laser ablation or double freeze-thaw cryotherapy.15 Disease progression can be noted by posterior subcapsular cataract and total retinal detachment. Pharmacologically, intravitreal or sub-Tenon corticosteroids and intravitreal anti-VEGF agents as individual or adjunctive therapy have been reported to reduce subretinal fluid, reduce macular exudation or nodule formation, and improve visual acuity in patients with Coats disease (Figure 2).16-19 Additionally, external drainage of subretinal fluid in additional to vitrectomy in patients with advanced cases of Coats disease was found to have better anatomical success when compared to vitrectomy alone.20 However, these reported cases are few, with minimal follow-up studies regarding long-term safety and efficacy.
In advanced stages of Coats disease, vitreoretinal surgical techniques may provide minimal maintenance of vision or prevent the need for enucleation. A preferred adjunct to ablative therapies is external subretinal fluid drainage.21,22 One study found that patients who underwent transscleral drainage of subretinal fluid (TDSRF) combined with pars plana vitrectomy (PPV) had anatomical success rate when compared to patients who underwent only TDSRF. Patients with TDSRF with PPV had lower incidence of epiretinal membrane formation and fewer subsequent laser photocoagulation procedures when compared to patients with TDSRF alone.23 Previous studies have investigated external drainage or vitrectomy and internal drainage of subretinal fluid followed by vasoablation.24 Another study found that the combination of PPV with external drainage, accompanied by scleral buckle, perfluoro-n-octane, and laser photocoagulation had complete anatomic attachment of stage 3B Coats-only detachments at the 6-month and 1-year follow-up.25 End-stage Coats disease with severe symptoms such as angle closure glaucoma may require enucleation.
CONCLUSION
Coats disease is a retinal vascular disorder more likely to present unilaterally in young males and is characterized by aneurysmal and telangiectatic retinal vessels with intraretinal or subretinal fluid. Staging systems exist based on the presence and location of exudation and degree of retinal detachment. An important distinction when making a diagnosis is to differentiate between Coats disease and retinoblastoma. Fundus autofluorescence and OCT are useful for diagnosis and treatment planning. Treatment is largely reserved for presentation of stage 2 and worse and can include laser photocoagulation and cryotherapy. Use of sub-Tenon corticosteroids, intravitreal anti-VEGF treatment, and surgical techniques, while effective in reported cases, require further study in efficacy. RP
REFERENCES
- Coats G. Forms of retinal diseases with massive exudation. Roy Lond Ophthalmol Hosp Rep. 1908;17:440-525.
- Schachat AP. Coats disease. In: Sadda SR, ed. Ryan’s Retina, 6th ed. Elsevier, 2018:1188-1199.
- Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: the 2000 Proctor Lecture. Am J Ophthalmol. 2001;131(5):572-583. doi:10.1016/s0002-9394(01)00896-0
- Shields CL, Udyaver S, Dalvin LA, et al. Coats disease in 351 eyes: analysis of features and outcomes over 45 years (by decade) at a single center. Indian J Ophthalmol. 2019;67(6):772-783. doi:10.4103/ijo.IJO_449_19
- Recchia FM. Coats disease. In: Hartnett ME, Trese M, Capone Jr. A, Keats BJB, Caputo G, eds. Pediatric Retina. 2nd ed. Wolters Kluwer Health; 2014:616-623.
- Jeng-Miller KW, Soomro T, Scott NL, et al. Longitudinal examination of fellow-eye vascular anomalies in Coats’ disease with widefield fluorescein angiography: a multicenter study. Ophthalmic Surg Lasers Imaging Retina. 2019;50(4):221-227. doi:10.3928/23258160-20190401-04
- Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131(5):561-571. doi:10.1016/s0002-9394(00)00883-7
- Gupta A, Paulbuddhe VS, Shukla UV, Tripathy K. Exudative retinitis (Coats disease). StatPearls. 2023.
- Toth CA, Ong SS. Coats disease and Coats plus syndrome. In: Toth CA, Ong SS, eds. Handbook of Pediatric Retinal OCT and the Eye-Brain Connection. Elsevier; 2020:149-153.
- Gupta MP, Dow E, Jeng-Miller KW, et al. Spectral domain optical coherence tomography findings in Coats disease. Retina. 2019;39(6):1177-1185. doi:10.1097/IAE.0000000000002120
- Ong SS, Cummings TJ, Vajzovic L, Mruthyunjaya P, Toth CA. Comparison of optical coherence tomography with fundus photographs, fluorescein angiography, and histopathologic analysis in assessing Coats disease. JAMA Ophthalmol. 2019;137(2):176-183. doi:10.1001/jamaophthalmol.2018.5654
- Ong SS, Buckley EG, McCuen BW 2nd, et al. Comparison of visual outcomes in Coats’ disease: a 20-year experience. Ophthalmology. 2017;124(9):1368-1376. doi:10.1016/j.ophtha.2017.03.051
- Gomez Morales A. Coats’ disease. Natural history and results of treatment. Am J Ophthalmol. 1965;60(5):855-865.
- Levinson JD, Hubbard GB 3rd. 577-nm yellow laser photocoagulation for Coats disease. Retina. 2016;36(7):1388-1394. doi:10.1097/IAE.0000000000000874
- Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of Coats disease. Surv Ophthalmol. 2014;59(1):30-46. doi:10.1016/j.survophthal.2013.03.007
- Patel NA, Berrocal AM, Murray TG, Villegas VM. Advanced Coats’ disease treated with intravitreal brolucizumab combined with laser photocoagulation. Am J Ophthalmol Case Rep. 2020;19:100815. doi:10.1016/j.ajoc.2020.100815
- Zhang L, Ke Y, Wang W, Shi X, Hei K, Li X. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats’ disease. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1339-1346. doi:10.1007/s00417-018-3949-1
- Oli A, Balakrishnan D, Jalali S. Coats’ disease: trends and long-term treatment outcomes in a tertiary referral centre. Ther Adv Ophthalmol. 2021;13:25158414211055957. doi:10.1177/25158414211055957
- Sein J, Tzu JH, Murray TG, Berrocal AM. Treatment of Coats’ disease with combination therapy of intravitreal bevacizumab, laser photocoagulation, and sub-Tenon corticosteroids. Ophthalmic Surg Lasers Imaging Retina. 2016;47(5):443-449. doi:10.3928/23258160-20160419-07
- Mano F, Matsushita I, Kondo H, Utamura S, Kondo C, Kusaka S. Vitrectomy and external drainage of subretinal fluid containing high concentration of vascular endothelial growth factor for advanced coats disease. Sci Rep. 2021;11(1):19333. doi:10.1038/s41598-021-98968-9
- Han ES, Choung HK, Heo JW, Kim SJ, Yu YS. The effects of external subretinal fluid drainage on secondary glaucoma in Coats’ disease. J AAPOS. 2006;10(2):155-158. doi:10.1016/j.jaapos.2005.12.001
- Yousef YA, ElRimawi AH, Nazzal RM, et al. Coats’ disease: characteristics, management, outcome, and scleral external drainage with anterior chamber maintainer for stage 3b disease. Medicine (Baltimore). 2020;99(16):e19623. doi:10.1097/MD.0000000000019623
- Ucgul AY, Ozdek S, Ertop M, Atalay HT. External drainage alone versus external drainage with vitrectomy in advanced Coats disease. Am J Ophthalmol. 2021;222:6-14. doi:10.1016/j.ajo.2020.09.006
- Li AS, Capone A Jr, Trese MT, et al. Long-term outcomes of total exudative retinal detachments in stage 3B Coats disease. Ophthalmology. 2018;125(6):887-893. doi:10.1016/j.ophtha.2017.12.010
- Rao P, Knapp AN, Todorich B, Drenser KA, Trese MT, Capone A Jr. Anatomical surgical outcomes of patients with advanced coats disease and coats-like detachments: review of literature, novel surgical technique, and subset analysis in patients with facioscapulohumeral muscular dystrophy. Retina. 2019;39 Suppl 1:S182-S190. doi:10.1097/IAE.0000000000002463
- Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: an overview of classification, management and outcomes. Indian J Ophthalmol. 2019;67(6):763-771. doi:10.4103/ijo.IJO_841_19
- Aerts I, Lumbroso-Le Rouic L, Gauthier-Villars M, Brisse H, Doz F, Desjardins L. Retinoblastoma. Orphanet J Rare Dis. 2006;1:31. doi:10.1186/1750-1172-1-31
- de Graaf P, Göricke S, Rodjan F, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol. 2012;42(1):2-14. doi:10.1007/s00247-011-2201-5
- Razek AA, Elkhamary S. MRI of retinoblastoma. Br J Radiol. 2011;84(1005):775-784. doi:10.1259/bjr/32022497








