Diabetic retinopathy (DR) is one of the leading causes of blindness worldwide, primarily affecting individuals aged 20 to 74 years.1 For decades, the gold standard for assessing diabetic retinopathy was the Early Treatment of Diabetic Retinopathy Study (ETDRS) classification system, which is based on grading stereo photographs of the 7 standard fields (7F) and classifying DR into 13 complex levels. Although this grading scale has served as an excellent tool in several epidemiologic studies and clinical trials, its applicability is limited due to its complexity.2 Furthermore, the 7F protocol is not convenient for patients, considering that it is time consuming and requires pupil dilatation as well as patient cooperation.
In 2003, a panel of experts created a simplified classification system called the International Clinical Diabetic Retinopathy (ICDR) severity scale to simplify DR staging. This 5-step scale is simple and easy to remember, and it has become widely adopted in routine clinical practices.3
Although ETDRS and ICDR grading scales are well codified, and widely used, none of them consider changes in the retinal periphery because they capture only 30° to 50° of the retina.4 Thus, using these classification systems can be less relevant for assessing and documenting the progression in eyes with severe nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR), which are candidates to be treated with anti–vascular endothelial growth factor (anti-VEGF) agents.4 To overcome these limitations, ultrawidefield (UWF) imaging has emerged as a possible alternative, given that it enables a panoramic view to be obtained within a single shot, without the need for contact or the use of mydriatics.4
ULTRAWIDEFIELD IMAGING
Currently, the definition of UWF images is based on anatomic landmarks. According to the new consensus, UWF is a single-capture image system that is simultaneously centered on the fovea. It captures retinal anatomic features anterior to the vortex ampullae in all 4 quadrants (approximately 110° to 220°).5
The current landscape of UWF photography consists of several imaging systems, including the RetCam (Natus Medical Incorporated), Spectralis HRA (Heidelberg), Panoret-1000 (CMT Medical Technologies Inc), Clarus 500 (Carl Zeiss Meditec), Mirante (Nidek), and Optos. These devices have varying specifications and features in terms of field of view, multimodal capabilities, mechanism, image color, and need for contact lens. Yet, UWF imaging may be useful during the early stages of diagnosis, as well as in diabetic patients who tend to have poor dilatation.
CASE PRESENTATION
A 71-year-old female patient was diagnosed with diabetes at age 44 and has been treated with insulin since she was 52. Initially this case would have been graded as mild NPDR, but the color UWF images highlighted several lesions, mainly in the retinal periphery, outside the EDTRS 7F (Figure 1).
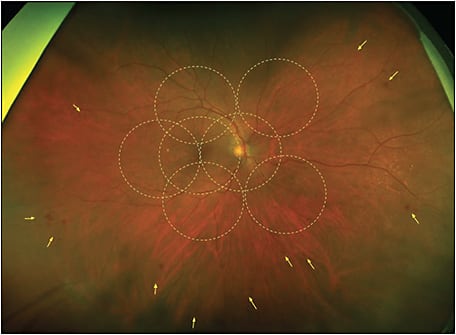
This patient was imaged with the Optos California UWF imaging device, which provides nonmydriatic panoramic fundus images with a 200° view of the retina, displaying 82% of retinal surface area in a single capture. Ultrawidefield fluorescein angiography (UWF-FA) showed microaneurysms and mild leakage at the posterior pole, and identified large areas of peripheral retinal nonperfusion (Figures 2 and 3). Given these results, the severity of this DR changed from mild to severe NPDR. The management was changed accordingly from follow-up to panretinal photocoagulation (PRP).
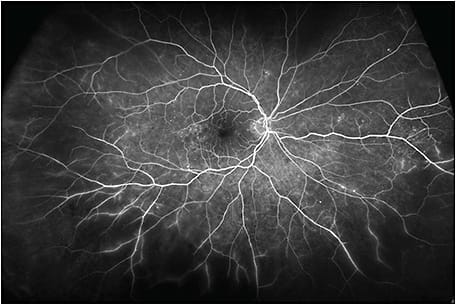
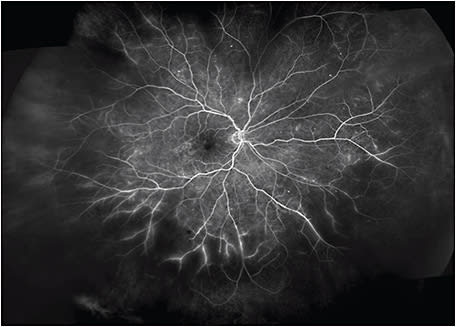
In this case, UWF fundus imaging enabled the detection of key lesions indicative of DR and large areas of retinal ischemia outside the 7F boundary. Based on the traditional grading scale, the severity of this case had initially been underestimated. However, UWF-FA data graded this DR as severe NPDR, which led to earlier treatment and closer follow-up.
DISCUSSION
To date, assessment of the severity of DR has been based on grading key DR lesions within the ETDRS 7F area with nearly 50° of visualization.4 Although it has been reliable for grading DR, this field of view excludes much of the peripheral retina. Moreover, montage images remain a burdensome and time-consuming process that require skilled photographers and patient cooperation.6
Silva et al introduced the concept of predominantly peripheral lesions (PPLs), which are the main lesions of DR (microaneurysms, hemorrhages, venous beading, intraretinal microvascular abnormalities, and neovascularization (NV)) spread over the peripheral retina outside of standard ETDRS fields.7-10 Numerous studies have underlined the clinical importance of these peripheral abnormalities. Silva et al found that eyes with PPL have a 3.2-fold increase in the risk of progression to PDR compared to eyes without PPL.10 Aiello et al found that 53.9% of new vessels elsewhere in 39 cases were located outside the area covered by the ETDRS 7 fields.8 Given that more than 50% of the graded lesions occur in the periphery, the likelihood of progressive and proliferative DR is greater in eyes with PPLs when using ETDRS 7F only.4,11
There is evidence that most of the growth factors that cause NV are preceded by an ischemic drive and patients with retinal ischemia are more likely to develop NV, macular ischemia, or treatment-resistant macular edema.12,13 In their 4-year prospective study, Silva et al found that baseline retinal nonperfusion and the presence of PPLs increase the risk of DR worsening.14 Overall, Aiello et al reported that using UWF imaging in clinical practice nearly doubled the frequency of DR detection,8 which would have otherwise gone undetected.
When used for treatment, UWF-FA may help design customized laser options for eyes with PDR. It has been suggested that targeted retinal photocoagulation may replace PRP to minimize PRP side effects and prevent excessive loss of the visual field.11 Thus, UWF imaging could also play a role in the management of DR, in addition to being a diagnostic tool. However, more randomized studies are needed to determine whether new treatment guided by peripheral retinal angiography data may improve patients’ outcomes.
CONCLUSION
Early detection and treatment of DR are crucial to help decrease the global burden of DR-related vision loss. Ultrawidefield imaging has revolutionized the way we evaluate and document the peripheral retina. It improves retinal visualization and makes it possible to detect more vascular pathology outside the ETDRS 7F. These findings appear to have prognostic significance and may therefore shape the management of diabetic patients. RP
REFERENCES
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124-136. doi:10.1016/S0140-6736(09)62124-3
- Solomon SD, Goldberg MF. ETDRS Grading of diabetic retinopathy: still the gold standard? Ophthalmic Res. 2019;62(4):190-195. doi:10.1159/000501372
- Wu L, Fernandez-Loaiza P, Sauma J, Hernandez-Bogantes E, Masis M. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4(6):290-294. doi:10.4239/wjd.v4.i6.290
- Ghasemi Falavarjani K, Tsui I, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy. Vision Res. 2017;139:187-190. doi:10.1016/j.visres.2017.02.009
- Patel SN, Shi A, Wibbelsman TD, Klufas MA. Ultra-widefield retinal imaging: an update on recent advances. Ther Adv Ophthalmol. 2020;12:2515841419899495. doi:10.1177/2515841419899495
- Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785-791. doi:10.1097/IAE.0b013e3182278b64
- Kumar V, Surve A, Kumawat D, et al. Ultra-wide field retinal imaging: a wider clinical perspective. Indian J Ophthalmol. 2021;69(4):824-835. doi:10.4103/ijo.IJO_1403_20
- Aiello LP, Odia I, Glassman AR, et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137(1):65-73. doi:10.1001/jamaophthalmol.2018.4982
- Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122(12):2465-2472. doi:10.1016/j.ophtha.2015.07.034
- Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949-956. doi:10.1016/j.ophtha.2015.01.008
- Cai S, Liu TYA. The role of ultra-widefield fundus imaging and fluorescein angiography in diagnosis and treatment of diabetic retinopathy. Curr Diab Rep. 2021;21(9):30. doi:10.1007/s11892-021-01398-0
- Değer Bilgeç M, Erol N, Topbaş S. Wide-field retinal imaging in adults and children. In: Nowinska A, ed. Novel Diagnostic Methods in Ophthalmology. IntechOpen; 2019. https://doi.org/10.5772/intechopen.84215.
- Liu TYA, Arevalo JF. Wide-field imaging in proliferative diabetic retinopathy. Int J Retina Vitreous. 2019;5(Suppl 1):20. doi:10.1186/s40942-019-0170-2
- Silva PS, Marcus DM, Liu D, et al. Association of ultra-widefield fluorescein angiography-identified retinal nonperfusion and the risk of diabetic retinopathy worsening over time [published correction appears in JAMA Ophthalmol. 2023 Jan 1;141(1):104]. JAMA Ophthalmol. 2022;140(10):936-945. doi:10.1001/jamaophthalmol.2022.3130








