The peripheral retina manifests a variety of vitreoretinal disorders, including retinal vascular diseases, peripheral retinal degenerations, uveitis, retinal or choroidal tumors, and hereditary retinal dystrophies, among others.1,2 The gold standard for evaluation of the retinal periphery is a dilated fundus exam (DFE) with scleral depression performed by an experienced retina specialist. Recent technological advances in widefield (WF) and ultrawidefield (UWF) cameras have enabled unparalleled imaging of the retinal periphery, in contrast to older technology that captured about 30° to 50° of the posterior pole (Figure 1) and into the midperiphery with sweep views.3

DEFINITION AND DEVICES
The Diabetic Retinopathy Clinical Research Network (DRCR Network) initially defined UWF as having at least a 100° view of the retina.4 The current consensus definition from the International Widefield Imaging Study Group states that WF imaging captures images of the retina posteriorly to the vortex vein ampullae in all 4 quadrants, whereas UWF is able to visualize retina anteriorly to vortex vein ampullae in all 4 quadrants in a single image captured centered on the fovea.5 Ultrawidefield imaging typically corresponds to a 110° to 220° field of view, visualizing up to 80% of the retina.6 Currently, no fundus camera is capable of capturing a “panretinal” image — with visualization from ora to ora (360 degrees) — in a single click, although multiple UWF images can be montaged together to create such an image.
The current landscape of UWF photography consists of several imaging systems that differ by need for contact lens, field of view, mechanism, multimodal capabilities, and type of image (color or pseudocolor). These include the RetCam (Natus Medical Incorporated), Spectralis HRA (Heidelberg), Panoret-1000 (CMT Medical Technologies Inc), Clarus 500 (Carl Zeiss Meditec), Mirante (Nidek), and Optos.
The RetCam, primarily used for neonatal and pediatric fundus imaging, is a multimodal imaging device that can view up to 130° of the retina. It is contact lens–based and uses a coaxial illumination system, so media opacities are more likely to result in poor image quality.7,8 It also has additional multimodal imaging capabilities for fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA). Another contact lens–based imaging system is the Spectralis HRA, which is a confocal scanning laser ophthalmoscope (SLO). An SLO uses beams of laser light to illuminate the retina, rather than bright flashes of light, and the confocal setup enables back-scattered light outside the focal plane to be blocked by a pinhole aperture.9 When used in conjunction with the Staurenghi 230 SLO Retina Contact Lens (Ocular Instruments Inc), it can provide up to a 150° field of view. The field of view without using a contact lens is 105°.10 The Spectralis can also take FFA, ICGA, and fundus autofluorescence (FAF) images.
Another contact-based fundus camera, Panoret-1000, used transscleral as opposed to transpupillary illumination, but this has since been commercially discontinued.11 In general, contact lens–based systems are good for patients who may not be able to position themselves, such as neonates and infants, but more patient cooperation and photographer skill are required to capture these images.
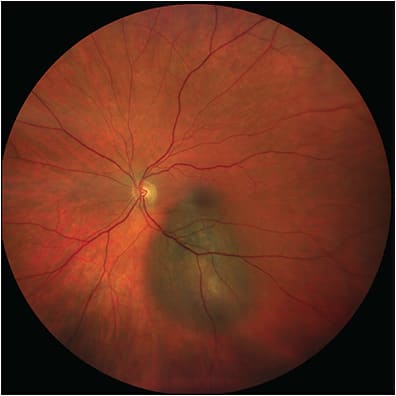
There are 3 currently available noncontact-based imaging systems. The Clarus 500 is a slit-scanning ophthalmoscope that uses broad-line fundus imaging — a combination of SLO and traditional fundus photography — to capture WF and fundus autofluorescence images.12 Its field of view is 133° in a single image, up to 200° for 2 images, or up to 267° for a montage of 6 images. It is most notable for its ability to take true-color images using 3 wide-spectrum light-emitting diodes to capture more accurate coloration of the fundus and reduce optic nerve head bleaching (Figure 2). It has multimodal imaging capabilities for FAF, and the Clarus 700 can also capture FFA images.
The Mirante is a multimodal imaging system using SLO that can view up to 163° of the retina. In addition to WF fundus photography, it is capable of FFA, ICGA, FAF (blue and green), optical coherence tomography, and optical coherence tomography angiography. It uses 4 separate lasers (blue, green, red, and infrared) which allow production of pseudocolor images that more closely resemble the actual color of the fundus. There is also a small coaxially placed pinhole that reduces backscattered and out-of-focus light.13
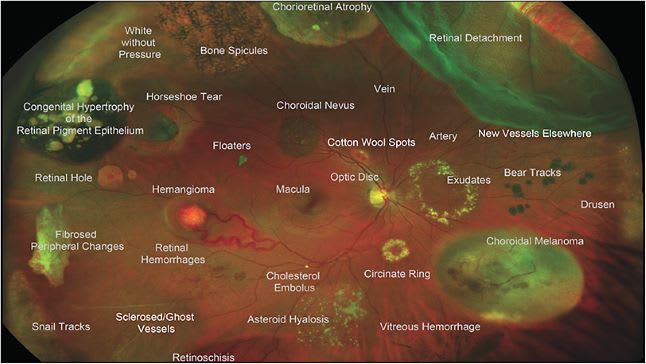
Finally, Optos (Optos Inc) is a noncontact SLO that can image up to 200° of the retina, or 82% of retinal surface area. Notably, it can acquire this image even through a nonmydriatic pupil by using ellipsoid mirror and virtual point technology.1 In addition to fundus photography, it can capture FFA, FAF (green), and ICGA images. Patients can be imaged sitting up or in the “flying baby” position in cases of retinopathy of prematurity (ROP) screening.14 Optos has the widest field of view in a single click of all existing cameras and does not require pupillary dilation, but its pseudocolor image can make it more difficult to detect the true coloration of retina, which can be problematic in imaging pathologies such as choroidal nevi.10 The composite image in Figure 3 shows how a variety of peripheral retinal pathologies would appear when captured on an UWF image.
ULTRAWIDEFIELD IN OPHTHALMOLOGY
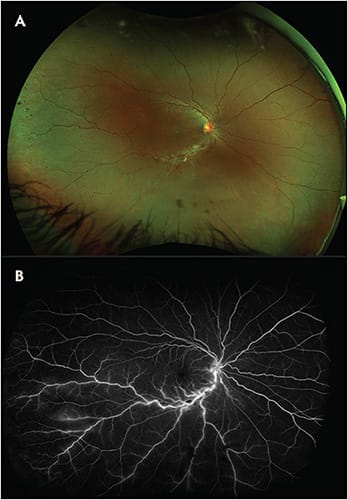
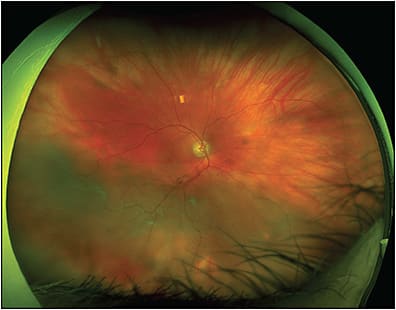
Multimodal UWF imaging is now widely used in ophthalmology clinics, especially in retina practices. Its numerous advantages include fast image acquisition time and simultaneous high-quality imaging of both the central and peripheral retina. Ultrawidefield imaging can aid in the diagnosis and management of both posterior and peripheral chorioretinal pathologies, including retinal vascular diseases (eg, diabetic retinopathy, venous or arterial occlusions, vasculitis) (Figure 4); age-related macular degeneration; retinal detachments (RD) (Figure 5); or peripheral retinal degenerations (eg, holes, lattice, retinoschisis); neoplastic lesions (primary malignancies or metastases); infectious and noninfectious posterior uveitis (Figure 6); hereditary chorioretinal dystrophies (Figure 7); and various pediatric vitreoretinal disorders such as ROP or familial exudative vitreoretinopathy.1,15 In addition, UWF imaging can monitor disease progression over time, provide patient education by helping patients visualize their own disease, and become an invaluable tool in telemedicine screening for diseases such as diabetic retinopathy.16
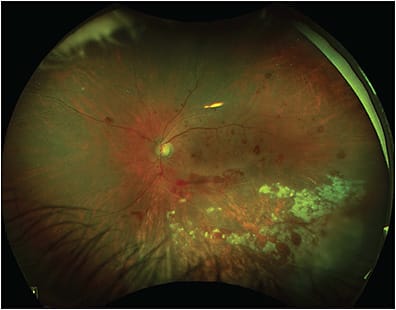
Given the widespread use of UWF imaging and its ability to capture a large part of the retinal periphery without the need for mydriasis (as in the Optos), it is reasonable to consider the possibility that nonmydriatic UWF imaging could replace the DFE in select asymptomatic patients, particularly return patients. Some patients do not tolerate dilating drops (especially children where instilling any eye drop is often challenging), and some patients are poor dilators.17,18 In addition, many patients find the associated light sensitivity and blurry vision after dilation to be particularly bothersome, especially when they have to come in for frequent appointments with a retina specialist. Finally, extended ophthalmoscopy is no longer reimbursed when color photos are also obtained. Thus, UWF imaging can be used as an initial screening tool for patients, who can be dilated afterward if necessary based on symptoms and imaging.
Several studies have been performed comparing nonmydriatic UWF to standard DFE in specific patient populations. For DR screening in asymptomatic patients, a study of 200 eyes showed that nonmydriatic UWF (Optos) was comparable to standard DFE and mydriatic 7-field Early Treatment Diabetic Retinopathy Study (ETDRS) photography in determining the severity of diabetic retinopathy and diabetic macular edema (DME), and the acquisition time for nonmydriatic UWF was significantly faster than ETDRS photograph.4 Another recent study of 322 diabetic eyes corroborated these findings, concluding that nonmydriatic UWF is comparable to standard DFE in identifying diabetic retinopathy.19 A few studies have also shown that there was no difference in diabetic retinopathy detection or grading when comparing nonmydriatic UWF to either mydriatic UWF imaging or standard fundus photography in multiple positions of gaze.20,21
For routine DFEs in asymptomatic patients after cataract surgery, 1 study of 184 eyes after cataract surgery compared detection of retinal lesions in nonmydriatic UWF, mydriatic slit-lamp exam with a Volk 90 diopter lens, or mydriatic Goldmann 3-mirror contact lens exam. It showed that the results obtained by UWF and dilated slit-lamp exam have good consistency and concluded that nonmydriatic UWF can be used as a screening tool to follow asymptomatic patients after cataract extraction.22 Another retrospective case series showed that nonmydriatic UWF imaging provided good visualization of the peripheral retina in more than 90% of patients when images were reviewed by a retina specialist.23 Other studies have confirmed that nonmydriatic UWF images are generally gradable — a study of 1,156 eyes in Japan showed that 99.7% of eyes imaged with Optos had sufficient image quality to detect retinal disease; only 4 of 1,156 images were unable to be graded due to media opacity from cataract or corneal pathology.24 Finally, a study comparing RD-related characteristics in rhegmatogenous RD patients who underwent nonmydriatic UWF imaging, DFE, and intraoperative evaluation showed there was no significant difference in detecting RD extent, assessment of macula, or percentage of detected retinal breaks between DFE and nonmydriatic UWF.3
LIMITATIONS
There are some limitations to nonmydriatic UWF imaging. As previously mentioned, the pseudocolor image of the Optos can make it difficult to assess true coloration of the retina.10 There is also some peripheral distortion and magnification leading to difficulty in measuring the area of lesions in the periphery.1 Nonmydriatic UWF may still miss some peripheral lesions, especially those that are in the superior and inferior periphery. The same study that showed high gradeability of Optos imaging also demonstrated that detection of peripheral retinal lesions such as chorioretinal atrophy and RD was significantly higher in the group that underwent dilation.24 Another study in a higher-risk population (1,725 myopic eyes being screened for intraocular contact lens surgery) showed that nonmydriatic UWF imaging had only 57% sensitivity for peripheral retinal holes and 65% for peripheral retinal degenerations compared to dilation and examination with Goldmann 3-mirror contact lens.25 Lastly, there are logistical considerations, such as the cost of obtaining and maintaining nonmydriatic UWF cameras, which may not make it accessible to every clinic.
CONCLUSION
Despite some limitations, nonmydriatic UWF imaging holds significant promise in potentially replacing the DFE in select asymptomatic patients. It is a powerful evaluation tool in retina clinics and can make it feasible to only dilate new patients, those with new symptoms, or those who have abnormalities detected on any type of multimodal imaging. This approach has the power to streamline busy retina clinics and spare dilation for asymptomatic patients with routine follow-up and stable imaging. Certain limitations can be mitigated with steered images (capturing UWF images in multiple directions of gaze), which has been shown to improve the sensitivity of detecting peripheral retinal lesions, or even simple maneuvers such as manually retracting the eyelid.26,27 Furthermore, the ability to obtain and transmit UWF imaging remotely has already made telemedicine screening for diabetic retinopathy a reality. In the future, such images could potentially even be obtained in patients’ homes, interpreted by an artificial intelligence algorithm, and sent to a retina specialist if abnormalities are found — similar to existing home OCT technologies (ie, Notal Vision Home AMD Monitoring Program) for early detection of macular neovascularization. Using such multimodal imaging technologies in the home and other remote settings can increase monitoring and early detection of treatable diseases while reducing the number of office visits. RP
REFERENCES
- Kumar V, Surve A, Kumawat D, et al. Ultra-wide field retinal imaging: a wider clinical perspective. Indian J Ophthalmol. 2021;69(4):824-835. doi:10.4103/ijo.IJO_1403_20
- Byer NE. The Peripheral Retina in Profile: A Stereoscopic Atlas. Criterion Press; 1982.
- Abadia B, Desco MC, Mataix J, et al. Non-mydriatic ultra-wide field imaging versus dilated fundus exam and intraoperative findings for assessment of rhegmatogenous retinal detachment. Brain Sci. 2020;10(8): doi:10.3390/brainsci10080521
- Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154(3):549-559.e2. doi:10.1016/j.ajo.2012.03.019
- Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3(10):843-849. doi:10.1016/j.oret.2019.05.007
- Patel SN, Shi A, Wibbelsman TD, Klufas MA. Ultra-widefield retinal imaging: an update on recent advances. Ther Adv Ophthalmol. 2020;12:2515841419899495. doi:10.1177/2515841419899495
- Shoughy SS, Arevalo JF, Kozak I. Update on wide- and ultra-widefield retinal imaging. Indian J Ophthalmol. 2015;63(7):575-581. doi:10.4103/0301-4738.167122
- Roth DB, Morales D, Feuer WJ, Hess D, Johnson RA, Flynn JT. Screening for retinopathy of prematurity employing the retcam 120: sensitivity and specificity. Arch Ophthalmol. 2001;119(2):268-272.
- Bille JF, ed. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Cham (CH): Springer; 2019.
- Callaway NF, Mruthyunjaya P. Widefield imaging of retinal and choroidal tumors. Int J Retina Vitreous. 2019;5(Suppl 1):49. doi:10.1186/s40942-019-0196-5
- Yao X, Son T, Ma J. Developing portable widefield fundus camera for teleophthalmology: technical challenges and potential solutions. Exp Biol Med (Maywood). 2022;247(4):289-299. doi:10.1177/15353702211063477
- Chen A, Dang S, Chung MM, et al. Quantitative comparison of fundus images by 2 ultra-widefield fundus cameras. Ophthalmol Retina. 2021;5(5):450-457. doi:10.1016/j.oret.2020.08.017
- Nagpal M, Mohan G, Soni A, Talati S, Mehrotra N. Widefield multimodal imaging. Retina Today. 2020(January/February).
- Patel CK, Fung TH, Muqit MM, et al. Non-contact ultra-widefield imaging of retinopathy of prematurity using the Optos dual wavelength scanning laser ophthalmoscope. Eye (Lond). 2013;27(5):589-596. doi:10.1038/eye.2013.45
- Tripathy K, Sharma YR, Gogia V, Venkatesh P, Singh SK, Vohra R. Serial ultra wide field imaging for following up acute retinal necrosis cases. Oman J Ophthalmol. 2015;8(1):71-72. doi:10.4103/0974-620X.149896
- Liu SL, Gonder JR, Owrangi E, et al. Effectiveness of nonmydriatic ultra-widefield retinal imaging to screen for diabetic eye disease: a randomized controlled trial (Clearsight). Diabetes Care. 2023;46(2):399-407. doi:10.2337/dc22-0713
- Tripathy K, Chawla R, Venkatesh P, Sharma YR, Vohra R. Ultrawide field imaging in uveitic non-dilating pupils. J Ophthalmic Vis Res. 2017;12(2):232-233. doi:10.4103/2008-322X.205360
- Tripathy K, Chawla R, Vohra R. Evaluation of the fundus in poorly dilating diabetic pupils using ultrawide field imaging. Clin Exp Optom. 2017;100(6):735-736. doi:10.1111/cxo.12484
- Szeto SKH, Wong R, Lok J, et al. Non-mydriatic ultrawide field scanning laser ophthalmoscopy compared with dilated fundal examination for assessment of diabetic retinopathy and diabetic macular oedema in Chinese individuals with diabetes mellitus. Br J Ophthalmol. Sep 2019;103(9):1327-1331. doi:10.1136/bjophthalmol-2018-311924
- Rasmussen ML, Broe R, Frydkjaer-Olsen U, et al. Comparison between Early Treatment Diabetic Retinopathy Study 7-field retinal photos and non-mydriatic, mydriatic and mydriatic steered widefield scanning laser ophthalmoscopy for assessment of diabetic retinopathy. J Diabetes Complications. 2015;29(1):99-104. doi:10.1016/j.jdiacomp.2014.08.009
- Mathis T, Lereuil T, Bruneteau L, et al. [Performance of ultra-wide field retinophotography for screening of diabetic retinopathy]. J Fr Ophtalmol. 2019;42(6):572-578. doi:10.1016/j.jfo.2019.02.008
- Peng J, Zhang Q, Jin HY, Lu WY, Zhao PQ. Ultra-wide field imaging system and traditional retinal examinations for screening fundus changes after cataract surgery. Int J Ophthalmol. 2016;9(9):1299-1303. doi:10.18240/ijo.2016.09.11
- Schwartz S, Gonzalez CL, Bhandari R, Oliver SN, Mandava N, Quiroz-Mercado H. Retina evaluation with nonmydriatic ultrawide-field color imaging after cataract extraction surgeries in asymptomatic patients. Ophthalmic Surg Lasers Imaging Retina. Jan 2015;46(1):50-55. doi:10.3928/23258160-20150101-08
- Kusumi Y, Sano M, Nakayama M, et al. [Efficacy of ultra-wide angle fundus imaging without dilated pupils in annual health check-up examination]. Nippon Ganka Gakkai Zasshi. Jan 2016;120(1):35-40.
- Yang D, Li M, Wei R, Xu Y, Shang J, Zhou X. Optomap ultrawide field imaging for detecting peripheral retinal lesions in 1725 high myopic eyes before implantable collamer lens surgery. Clin Exp Ophthalmol. Sep 2020;48(7):895-902. doi:10.1111/ceo.13809
- Li M, Yang D, Shen Y, et al. Application of mydriasis and eye steering in ultrawide field imaging for detecting peripheral retinal lesions in myopic patients. Br J Ophthalmol. 2022;doi:10.1136/bjophthalmol-2021-319809
- Cheng SC, Yap MK, Goldschmidt E, Swann PG, Ng LH, Lam CS. Use of the Optomap with lid retraction and its sensitivity and specificity. Clin Exp Optom. 2008;91(4):373-8. doi:10.1111/j.1444-0938.2007.00231.x








