Retinal vascular disorders often present in the first few years of life and are characterized by a genetic inheritance pattern that varies between diseases. While having different etiologic origins, developmental retinal vascular disorders, specifically familial exudative vitreoretinopathy (FEVR), Norrie disease, and incontinentia pigmenti (IP), share similarities between their clinical presentations, differential diagnosis, diagnostic criteria, and treatment plan.
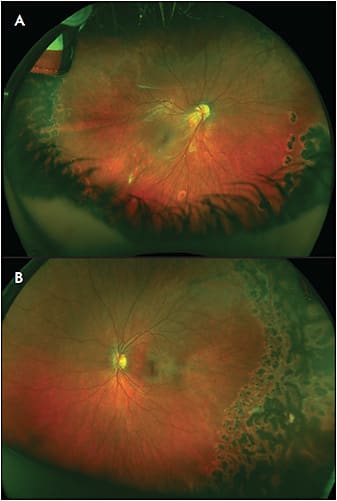
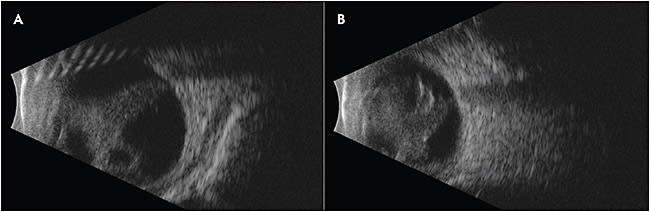
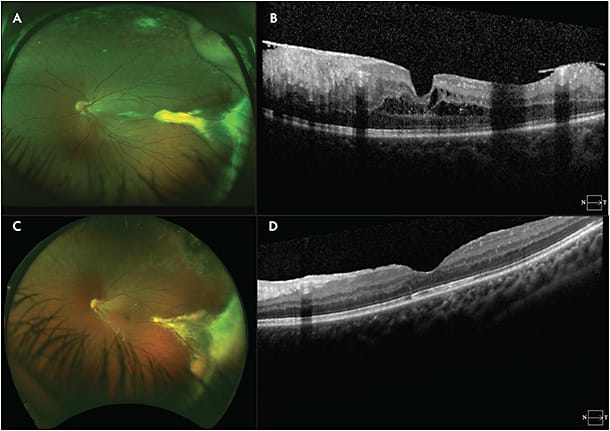
Familial exudative vitreoretinopathy is a genetically heterogeneous retinal vascular disorder characterized by failure of growth of peripheral retinal vasculature resulting in avascular retina (Figure 1).1 It was first described in 1969 by Criswick and Schepens as a form of retinopathy of prematurity in full-term infants,2 but it was later determined to be a distinct disease. Norrie disease, an X-linked recessive disorder, was initially described in 1927 by Norrie and later by Andersen and Warburg.3,4 The visual manifestations of Norrie disease are incomplete retinal vascularization and regression of primary hyaloid structures (Figure 2).5 Incontinentia pigmenti, also known as Bloch-Sulzberger syndrome, is an X-linked dominant (XD) disease first described in 1906 by Garrod (Figure 3).6 Incontinentia pigmenti is characterized by avascular peripheral retina; skin manifestations including diffuse blisters, erythema, and pale, hairless patches; and central nervous system (CNS) manifestations including seizures, epilepsy, and ischemic stroke. It affects females more often than males, given its inheritance pattern.7
GENETICS
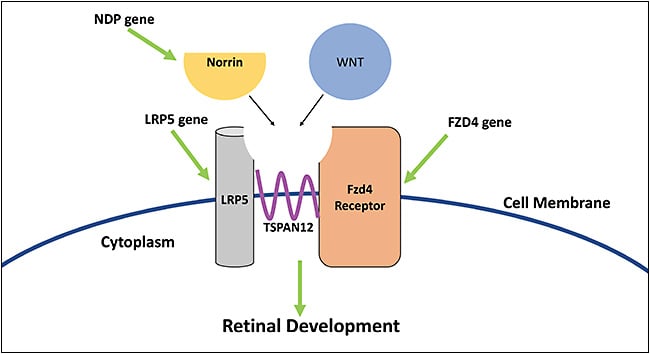
Familial exudative vitreoretinopathy is a genetically heterogenous disorder with autosomal dominant (AD), autosomal recessive (AR), or X-linked recessive pattern that exhibits variable penetrance across patients and, therefore, variable and asymmetric presentation. X-linked recessive disorders present at birth in males (Table 1).8 The Wnt-signaling and beta-catenin pathway are crucial for retinal development and are affected in FEVR and Norrie disease (Figure 4).9 The FZD4 gene, implicated in AD FEVR, produces the Fzd4 receptor. The LRP5 gene, also mutated in AD FEVR, produces LRP5 which is a co-receptor of Fzd4 in the Wnt pathway. The NDP gene is mutated in Norrie disease and in X-linked FEVR, and produces norrin, which is a ligand of the Fzd4 receptor. These deviations in the Wnt pathway cause downstream dysregulation of retinal development, which manifests as FEVR and Norrie disease.10,11
| FAMILIAL EXUDATIVE VITREORETINOPATHY (FEVR) | NORRIE DISEASE | INCONTINENTIA PIGMENTI | |
| Gender prevalence | No preference | Male more often than female | Female more often than male |
| Common Inheritance pattern | Autosomal dominant (most common), autosomal recessive, or X-linked recessive | X-linked recessive | X-linked dominant |
| Common genes involved | Frizzled-4 (FZD4, 11q14.2), Norrie disease pseudoglioma (NDP), Low-density lipoprotein receptor related protein-5 (LRP5), Tetraspanin-12 (TSPAN12), Zinc finger protein-408 (ZNF408). About 50% of all FEVR cases worldwide have known pathologic variant. | NDP | Inhibitor of nuclear factor kappa B kinase regulatory subunit gamma (IKBKG) |
Incontinentia pigmenti is extremely rare, with an estimated 27.6 new cases per year worldwide.12 It has an XD inheritance pattern and primarily presents in female patients due to lethality of homozygosity in utero.13 Male inheritance forms of IP have been reported as cases of Klinefelter disease or somatic mosaicism.14,15 The NF-KB pathway is involved in regulation of genes controlling cell survival, apoptosis regulation, and immune response. In IP, mutations in the IKBKG gene resulted in shortened amino-acid proteins which are unable to elicit an adequate NF-KB response, leading to the retinal and dermatologic manifestations seen in IP.16,17
CLINICAL PRESENTATION
Initial presentations and retinal manifestations of FEVR, Norrie disease, and IP are found in Table 2. Retinal folds extending from the posterior pole to the peripheral retina are seen in an estimated one-third of patients with FEVR.1 Familial exudative vitreoretinopathy can present with asymmetric but bilateral signs and symptoms.18,19 Although FEVR can present at any age, patients usually have a mean onset of 5 years of age,19 with earlier presentations, especially in the first year of life, carrying a poorer prognosis.1 A clinical staging system was developed in 1998, and an update was proposed in 2014, including fluorescein angiography findings (Table 3).18,20
| FAMILIAL EXUDATIVE VITREORETINOPATHY | NORRIE DISEASE | INCONTINENTIA PIGMENTI | |
| Presenting symptoms | Reduced visual acuity, strabismus, leukocoria | Blindness shortly after birth, leukocoria, gray/yellow pseudoglioma, iris atrophy, progressive eye shrinkage | Reduced visual acuity, strabismus, leukocoria |
| Retinal manifestations | Peripheral vascular abnormalities with presence of vascular buds at vascular-avascular boundary, subretinal exudation, vitreoretinal traction, retinal folds, retinal detachments | Dysplastic retina, retinal folds, vitreous hemorrhage, retinal detachments | Avascular peripheral retina with remodeling and collaterals at the vascular-avascular boundary, foveal hypoplasia, retinal detachments |
| Systemic manifestations | NA | Hearing loss, developmental delay | Skin lesions, retinal detachments, dental anomalies, seizures, ischemic stroke, nail dystrophy, alopecia |
| STAGING OF FAMILIAL EXUDATIVE VITREORETINOPATHY | |
| Stage 1 | Avascular periphery |
| Stage 2a | Retinal neovascularization without exudation |
| Stage 2b | Retinal neovascularization with exudation |
| Stage 3a | Extramacular retinal detachment without exudation |
| Stage 3b | Extramacular retinal detachment with exudation |
| Stage 4a | Macula-involving retinal detachment with exudation |
| Stage 4b | Macula-involving retinal detachment with exudation |
| Stage 5 | Total retinal detachment |
Due to the X-linked recessive pattern of inheritance, Norrie disease almost entirely affects males, with very rare reports of female disease, accounted for by possible lyonization.21,22 Affected males transmit pathologic variant to their daughters, who will be disease carriers, and sons are not affected. Norrie disease often results in bilateral blindness at or shortly after birth in male patients. Progressive eye shrinkage can be observed over time with increasing traction between the posterior retina and posterior lens capsule. Patients can develop cataracts and opaque corneas within the first decade of life.8 Nonocular manifestations are particularly distinct in Norrie disease, with nearly 30% of patients developing sensorineural hearing loss and CNS symptoms including developmental delay.23
Incontinentia pigmenti can manifest in patients at birth or shortly thereafter as strabismus, likely due to visual input deprivation from lens or retinal manifestations of disease.24 Patients can develop cataracts and microphthalmos as well.25 Although visual symptoms may be present, nonocular involvement is more extensive and characteristic in IP. Dermatologic manifestations of IP present at birth as diffuse rash with erythema, vesicles, and eosinophilia with truncal hyperpigmentation.7 Dental manifestations are common in patients and include delayed eruption and missing teeth.26 Central nervous system manifestations can contribute significantly to patient morbidity, with nearly 30% of patients experiencing CNS symptoms including ischemic stroke, hemiplegia, developmental delay, and encephalopathy.27,28 The most common CNS manifestation is seizures, which often present in the first week of life.
DIFFERENTIAL DIAGNOSIS
Familial exudative vitreoretinopathy, Norrie disease, and IP share certain clinical manifestations, making it difficult to differentiate the 3 diseases. Differential diagnosis can include retinopathy of prematurity, Coats disease, persistent fetal vasculature, and retinoblastoma.1,7,8 Many of these diseases can share features such as peripheral retinal nonperfusion or leukocoria. Family history can be helpful in determining an inheritance pattern specific to each disease. Nonocular manifestations are particularly useful in distinguishing these diseases as well. Incontinentia pigmenti will have characteristic skin manifestations, and Norrie disease can present with sensorineural hearing, which can be helpful in finalizing a diagnosis.
DIAGNOSTIC TESTING
Indirect ophthalmoscopy is an important tool in the diagnosis of FEVR, Norrie disease, and IP. However, fluorescein angiography, B-scan ultrasound, and genetic testing are critical adjuncts for diagnosis and treatment planning. Fluorescein angiography can identify abnormal macular perfusion and remodeling at the fovea, as well as extensive peripheral nonperfusion. In the presence of cataracts or vitreous hemorrhage, ultrasound can be used.
Microstructural anomalies can be identified on optical coherence tomography in patients with FEVR and IP. Reported findings in FEVR include posterior hyaloid organization and traction on macular and optic nerve, foveal anatomical changes, cystoid macular edema, and subretinal lipid aggregation29,30 and inner and outer macular thinning in IP.31,32 Optical coherence tomography angiography can also be used as diagnostic testing. Studies have demonstrated marked macular optical coherence tomography angiography abnormalities in FEVR patients, including vessel dilation, disorganized vessel pattern, areas of decreased vessel density, and stub-like vessel terminations.33,34
Genetic testing is generally indicated for all patients and helpful for diagnosis, especially with Norrie disease and IP. Genetic deletion accounts for nearly 80% of all disease in IP.7 Family history is also helpful in making the diagnosis. It is strongly recommended that patients with clinical symptoms consistent with FEVR, Norrie disease, or IP be offered genetic testing and genetic counseling. In addition, testing for sensorineural hearing loss in Norrie disease or skin biopsy in IP can be helpful for diagnosis.
TREATMENT
Treatment for FEVR includes ablation via laser therapy of the avascular retina if vascular irregularity is observed at the avascular-vascular boundary.1 Treatment earlier in disease course generally yields better visual results, especially with laser therapy, which is more effective if used prior to the development of subretinal fluid.35 Surgical intervention can be required for severe FEVR with vitreous hemorrhage, exudation, and tractional retinal detachment.1,36 Anti-VEGF agents can also be indicated in FEVR cases with exudation and possibly hemorrhage.37,38
Norrie disease does not have a treatment, but there are therapeutic measures that can be taken to preserve as much vision as possible. While there are reports of laser photocoagulation in early stages of Norrie disease, ablative therapy is not commonly used.39 Surgical intervention involves vitrectomy, which is believed to encourage normal ocular development by releasing vitreous traction connecting the posterior lens to the retina.8,40 Earlier surgical intervention is linked to improved outcomes in maintaining at least light perception in eyes. One group found that planned preterm delivery at 34 weeks’ gestation combined with laser ablation of avascular retina and intravitreal bevacizumab resulted in BCVA of 20/80, rudimentary foveal development, and absence of retinal detachments at an 8-year follow-up time point.41
Peripheral retinal vasculature abnormalities in IP are generally treatable through ablative therapy via laser photocoagulation or cryotherapy, which can stop progression of IP due to epiretinal neovascularization, exudation, or fibrosis.42-44 There are reports of anti-VEGF injections being used for management of IP, with unclear consensus on the safety and efficacy.45-48 Ultimately, treatment and management are multidisciplinary and lifelong. If retinal vasculature is normal after infancy, routine follow-up appointments can be appropriate.
CONCLUSION
Familial exudative vitreoretinopathy, Norrie disease, and IP, while all categorized as developmental retinal vascular disorders, are distinguishable through various factors (Table 2). Familial exudative vitreoretinopathy and IP both have avascular regions of the retina, with their avascular-vascular boundaries displaying vascular buds and collaterals, respectively. Norrie disease presents with dysplastic retina with a gray/yellow pseudoglioma appearance. The 3 diseases each have a distinct genetic inheritance pattern, meaning that genetic testing should be used in their diagnosis. An effective way to distinguish the 3 diseases is through their nonocular manifestations, with Norrie disease presenting with hearing loss and developmental delay, and IP presenting with skin lesions, dental anomalies, nail dystrophy, and seizures. Diagnosis can be completed by visualization of the retina via indirect ophthalmoscopy, fluorescein angiography, optical coherence tomography, and optical coherence tomography angiography, but ultimately, genetic testing is critical to confirming an etiology. Familial exudative vitreoretinopathy and IP are shown to respond to laser therapy used in earlier disease stages, while Norrie disease primarily relies on vitrectomy for vision preservation. RP
REFERENCES
- Hartnett ME, Capone A, Keats BJB, Caputo G. Familial exudative vitreoretinopathy (FEVR). In Hartnett ME, Capone A, Keats BJB, Caputo G, eds. Pediatric Retina. 2nd ed. Wolters Kluwer Health; 2014.
- Criswick VG, Schepens CL. Familial exudative vitreoretinopathy. Am J Ophthalmol. 1969;68(4):578-594. doi:10.1016/0002-9394(69)91237-9
- Warburg M. Norrie’s disease. Trans Ophthalmol Soc U K (1962). 1965;85:391-408.
- Andersen SR, Warburg M. Norrie’s disease: congenital bilateral pseudotumor of the retina with recessive X-chromosomal inheritance; preliminary report. Arch Ophthalmol. 1961;66:614-618. doi:10.1001/archopht.1961.00960010616003
- Sadda SR, Schachat AP, Wilkinson CP, Hinton DR, Wiedemann P. Pediatric retinal vascular diseases. In Sadda SR, Schachat AP, Wilkinson CP, Hinton DR, Wiedemann P, eds. Ryan’s Retina. 6th ed. Elsevier; 2018:1246-1267.
- Garrod AE. Peculiar pigmentation of the skin in an infant. Trans Clin Soc (London). 1906;39:216.
- Hartnett ME, Capone A, Keats BJB, Caputo G. Incontinentia pigmenti. In Hartnett ME, Capone A, Keats BJB, Caputo G, eds. Pediatric Retina. 2nd ed. Wolters Kluwer Health; 2014:354-361.
- Hartnett ME, Capone A, Keats BJB, Caputo G. Norrie disease. In Hartnett ME, Capone A, Keats BJB, Caputo G, eds. Pediatric Retina. 2nd ed. Wolters Kluwer Health; 2014:350-353.
- Panagiotou ES, Sanjurjo Soriano C, Poulter JA, Lord EC, Dzulova D, Kondo H, et al. Defects in the Cell Signaling Mediator β-Catenin Cause the Retinal Vascular Condition FEVR. Am J Hum Genet. 2017;100(6):960-8.
- Black G, Redmond RM. The molecular biology of Norrie’s disease. Eye (Lond). 1994;8 (Pt 5):491-496. doi:10.1038/eye.1994.124
- Warden SM, Andreoli CM, Mukai S. The Wnt signaling pathway in familial exudative vitreoretinopathy and Norrie disease. Semin Ophthalmol. 2007;22(4):211-217. doi:10.1080/08820530701745124
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31(6):e45-e52. doi:10.1016/j.pedhc.2017.07.003
- Berlin AL, Paller AS, Chan LS. Incontinentia pigmenti: a review and update on the molecular basis of pathophysiology. J Am Acad Dermatol. 2002;47(2):169-190. doi:10.1067/mjd.2002.125949
- Buinauskaite E, Buinauskiene J, Kucinskiene V, Strazdiene D, Valiukeviciene S. Incontinentia pigmenti in a male infant with Klinefelter syndrome: a case report and review of the literature. Pediatr Dermatol. 2010;27(5):492-495. doi:10.1111/j.1525-1470.2010.01261.x
- Kenwrick S, Woffendin H, Jakins T, et al. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter syndrome. Am J Hum Genet. 2001;69(6):1210-1217. doi:10.1086/324591
- Smahi A, Courtois G, Rabia SH, et al. The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11(20):2371-2375. doi:10.1093/hmg/11.20.2371
- Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405(6785):466-472. doi:10.1038/35013114
- Pendergast SD, Trese MT. Familial exudative vitreoretinopathy. Results of surgical management. Ophthalmology. 1998;105(6):1015-1023. doi:10.1016/S0161-6420(98)96002-X
- Ranchod TM, Ho LY, Drenser KA, Capone A Jr, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011;118(10):2070-2075. doi:10.1016/j.ophtha.2011.06.020
- Kashani AH, Learned D, Nudleman E, Drenser KA, Capone A, Trese MT. High prevalence of peripheral retinal vascular anomalies in family members of patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(1):262-268. doi:10.1016/j.ophtha.2013.08.010
- Sims KB, Irvine AR, Good WV. Norrie disease in a family with a manifesting female carrier. Arch Ophthalmol. 1997;115(4):517-519. doi:10.1001/archopht.1997.01100150519012
- Shastry BS, Hiraoka M, Trese DC, Trese MT. Norrie disease and exudative vitreoretinopathy in families with affected female carriers. Eur J Ophthalmol. 1999;9(3):238-242. doi:10.1177/112067219900900312
- Warburg M. Norrie’s disease. A congenital progressive oculo-acoustico-cerebral degeneration. Acta Ophthalmol (Copenh). 1966;1-47.
- Goldberg MF. The skin is not the predominant problem in incontinentia pigmenti. Arch Dermatol. 2004;140(6):748-750. doi:10.1001/archderm.140.6.748
- Hadj-Rabia S, Froidevaux D, Bodak N, et al. Clinical study of 40 cases of incontinentia pigmenti. Arch Dermatol. 2003;139(9):1163-1170. doi:10.1001/archderm.139.9.1163
- Minić S, Trpinac D, Gabriel H, Gencik M, Obradović M. Dental and oral anomalies in incontinentia pigmenti: a systematic review. Clin Oral Investig. 2013;17(1):1-8. doi:10.1007/s00784-012-0721-5
- Meuwissen ME, Mancini GM. Neurological findings in incontinentia pigmenti; a review. Eur J Med Genet. 2012;55(5):323-331. doi:10.1016/j.ejmg.2012.04.007
- Carney RG. Incontinentia pigmenti. A world statistical analysis. Arch Dermatol. 1976;112(4):535-542.
- Yonekawa Y, Thomas BJ, Drenser KA, Trese MT, Capone A Jr. Familial exudative vitreoretinopathy: spectral-domain optical coherence tomography of the vitreoretinal interface, retina, and choroid. Ophthalmology. 2015;122(11):2270-2277. doi:10.1016/j.ophtha.2015.07.024
- Lee J, El-Dairi MA, Tran-Viet D, et al. Longitudinal changes in the optic nerve head and retina over time in very young children with familial exudative vitreoretinopathy. Retina. 2019;39(1):98-110. doi:10.1097/IAE.0000000000001930
- Mangalesh S, Chen X, Tran-Viet D, Viehland C, Freedman SF, Toth CA. Assessment of the retinal structure in children with incontinentia pigmenti. Retina. 2017;37(8):1568-1574. doi:10.1097/IAE.0000000000001395
- Basilius J, Young MP, Michaelis TC, Hobbs R, Jenkins G, Hartnett ME. Structural abnormalities of the inner macula in incontinentia pigmenti. JAMA Ophthalmol. 2015;133(9):1067-1072. doi:10.1001/jamaophthalmol.2015.1700
- Hsu ST, Finn AP, Chen X, et al. Macular microvascular findings in familial exudative vitreoretinopathy on optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):322-329. doi:10.3928/23258160-20190503-11
- Hsu ST, Chen X, Ngo HT, et al. Imaging infant retinal vasculature with OCT angiography. Ophthalmol Retina. 2019;3(1):95-96. doi:10.1016/j.oret.2018.06.017
- Shukla D, Singh J, Sudheer G, et al. Familial exudative vitreoretinopathy (FEVR). Clinical profile and management. Indian J Ophthalmol. 2003;51(4):323-328.
- Hocaoglu M, Karacorlu M, Sayman Muslubas I, Ersoz MG, Arf S. Anatomical and functional outcomes following vitrectomy for advanced familial exudative vitreoretinopathy: a single surgeon’s experience. Br J Ophthalmol. 2017;101(7):946-950. doi:10.1136/bjophthalmol-2016-309526
- Lu YZ, Deng GD, Liu JH, Yan H. The role of intravitreal ranubizumab in the treatment of familial exudative vitreoretinopathy of stage 2 or greater. Int J Ophthalmol. 2018;11(6):976-980. doi:10.18240/ijo.2018.06.13
- Quiram PA, Drenser KA, Lai MM, Capone A Jr, Trese MT. Treatment of vascularly active familial exudative vitreoretinopathy with pegaptanib sodium (Macugen) [published correction appears in Retina. 2009 Jan;29(1):127]. Retina. 2008;28(3 Suppl):S8-S12. doi:10.1097/IAE.0b013e3181679bf6
- Chow CC, Kiernan DF, Chau FY, et al. Laser photocoagulation at birth prevents blindness in Norrie’s disease diagnosed using amniocentesis. Ophthalmology. 2010;117(12):2402-2406. doi:10.1016/j.ophtha.2010.03.057
- Walsh MK, Drenser KA, Capone A Jr, Trese MT. Early vitrectomy effective for Norrie disease. Arch Ophthalmol. 2010;128(4):456-460. doi:10.1001/archophthalmol.2009.403
- Sisk RA, Miraldi-Utz V, Schwartz TL, Hufnagel RB, Ahmed ZM. Long-term anatomic and visual outcomes of planned preterm delivery and treatment of Norrie disease. Ophthalmic Surg Lasers Imaging Retina. 2022;53(8):464-467. doi:10.3928/23258160-20220706-01
- Nguyen JK, Brady-Mccreery KM. Laser photocoagulation in preproliferative retinopathy of incontinentia pigmenti. J AAPOS. 2001;5(4):258-259. doi:10.1067/mpa.2001.117098
- Batioglu F, Ozmert E. Early indirect laser photocoagulation to induce regression of retinal vascular abnormalities in incontinentia pigmenti. Acta Ophthalmol. 2010;88(2):267-268. doi:10.1111/j.1755-3768.2008.01394.x
- Nakao S, Nishina S, Tanaka S, Yoshida T, Yokoi T, Azuma N. Early laser photocoagulation for extensive retinal avascularity in infants with incontinentia pigmenti. Jpn J Ophthalmol. 2020;64(6):613-620. doi:10.1007/s10384-020-00768-7
- Kunzmann S, Ngyuen T, Stahl A, et al. Necrotizing enterocolitis after intravitreal bevacizumab in an infant with Incontinentia Pigmenti - a case report. BMC Pediatr. 2019;19(1):353. Published 2019 Oct 15. doi:10.1186/s12887-019-1732-z
- Wang X, Liang JH. [Treatment of retinopathy of incontinentia pigmenti by anti-vascular endothelial growth factor]. Zhonghua Yan Ke Za Zhi. 2019;55(4):294-301. doi:10.3760/cma.j.issn.0412-4081.2019.04.012
- Liang L, Yang Y, Bu S, Lu F. Case report: a case of cotton-wool spots after intravitreal injection of conbercept in an infant with incontinentia pigmenti. Front Med (Lausanne). 2021;8:761398. Published 2021 Dec 21. doi:10.3389/fmed.2021.761398
- Lin KL, Hirose T, Kroll AJ, Lou PL, Ryan EA. Prospects for treatment of pediatric vitreoretinal diseases with vascular endothelial growth factor inhibition. Semin Ophthalmol. 2009;24(2):70-76. doi:10.1080/08820530902800108








