Radiation retinopathy (RR) is a well-known complication of radiation therapy, which is often used in the treatment of uveal melanomas, as well as head and neck malignancies. An exposure can occur from any source of radiation, including external beam radiotherapy, plaque brachytherapy, proton beam radiation, helium ion radiotherapy, and gamma knife radiotherapy. Scatter of radioactive particles lead to progressive endothelial cell damage within the retinal vasculature. This results in increased vascular permeability, leukocyte adhesion, and thrombosis, ultimately contributing to capillary nonperfusion (CNP), macular edema, neovascularization, and hemorrhage.1 Chronic inflammation and oxidative stress can also lead to damage to the choriocapillaris, retinal pigment epithelium (RPE), photoreceptor cells, and the retinal nerve fiber layer, leading to secondary changes in the retinal vasculature. Ultimately, vision loss in RR can result from macular edema (ME) or proliferative radiation retinopathy (PRR) and its associated late complications.
RISK FACTORS
The severity and onset of radiation retinopathy depend on several patient-level risk factors, including younger age, pre-existing ocular conditions, and pre-existing systemic diseases, such as diabetes, hypertension, coronary artery disease, and history of chemotherapy.2 Diabetes can provide a synergistic effect on retinal capillaries augmenting the damage done by RR and increasing the risk of visual loss by nearly 300%.3 Other malignancy-related associations with the incidence of RR include the total dose and fractionation of radiation, as well as the location and size of the treated tumor. The development of RR has been found to occur more frequently at total doses greater than 45 Gy.4 Proliferative radiation retinopathy has been reported in 7% of patients with plaque radiation for uveal melanoma within 15 years, with a mean time to a PRR diagnosis of 32 months.5
DIAGNOSIS
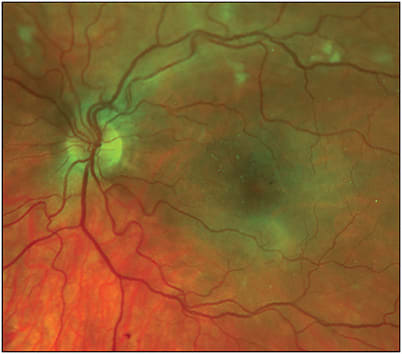
The clinical presentation of RR is variable and can range from asymptomatic to severe visual impairment. The fundus may resemble stages of diabetic retinopathy, vascular occlusion, or hypertensive retinopathy. Typical fundus findings include first the appearance of microaneurysms followed by telangiectasias, hard exudates, perivascular sheathing, CNP, cotton wool spots, retinal hemorrhages, and ME. These changes can occur variably and progress over time, leading to further retinal ischemia and neovascularization (ie, PRR). Additional late complications include vitreous hemorrhage, neovascular glaucoma, and rarely tractional retinal detachments. Radiation retinopathy can be differentiated from other vascular conditions such as diabetic retinopathy based on history of prior radiation, presence of RPE atrophy, or unilateral presentation.
Diagnostic imaging, including fluorescein angiography, may identify additional areas of macular leakage, CNP, and neovascularization not seen readily on exam, which has been shown to be associated with a worse visual prognosis.6 Optical coherence tomography is useful for identifying thinning of the inner plexiform, inner nuclear, and outer plexiform layers as well as ME and outer retinal damage, including ellipsoid zone loss. Optical coherence tomography angiography can be helpful for detecting signs of vascular compromise including microaneurysms, CNP, and neovascularization.
MANAGEMENT
Treatment for RR is tailored to individual patient findings, and management is aimed at preventing further visual loss and optimizing visual function. Treatment options include anti–vascular endothelial growth factor (VEGF) therapy, laser photocoagulation, and surgical intervention. There are currently no drugs specifically approved by the US Food and Drug Administration for the treatment of RR. However, some drugs that have shown efficacy, although used off label, include anti-VEGF agents and corticosteroids.
Historically, focal laser was the primary treatment for ME secondary to RR.7-9 However, in the age of anti-VEGF agents, therapy now commonly consists of intravitreal injections. In a prospective study of 120 consecutive patients with RR, anti-VEGF therapy was found to be effective in reducing ME, and 80% remained within 2 lines or better of presenting visual acuity.10 Another study found that ranibizumab was comparable to focal laser with or without peripheral laser treatment at year 1 follow-up in terms of best-corrected visual acuity and macular thickness.11
However, unlike ME from other conditions such as diabetes, anti-VEGF treatment duration appears to be shorter in ME due to RR. One randomized control trial demonstrated that aflibercept (Eylea; Regeneron) treatment for radiation maculopathy may be required every 6 weeks, with difficulty extending the treatment interval beyond 6 weeks.12 Another study evaluating ranibizumab (Lucentis; Genentech) dosing regimens found at 2 years of follow-up that treat-and-extend regimens may not be as efficacious as monthly dosing.13
Corticosteroids, including long-acting fluocinolone acetonide 0.19 mg (Iluvien; Alimera Sciences) and dexamethasone 0.7 mg implants (Ozurdex; Allergan/AbbVie), have also been used to treat RR. A study of a small sample of 8 patients demonstrated that both ranibizumab injections and dexamethasone implants were able to achieve improved mean visual acuity and decreased foveal thickness.14 However, several studies suggest that use of steroid agents may be more useful as adjunctive therapies to anti-VEGF agents rather than primary therapy, especially in patients with incomplete response or contraindications to anti-VEGF therapy.15 In light of potential ocular comorbidities, like malignancy-related glaucoma and radiation-induced cataracts, consideration should be given to the potential of steroids exacerbating these conditions.
Proliferative radiation retinopathy occurs as neovascular fronds grow from neovascular vessels into the hyaloid scaffold, causing traction. Similar to other proliferative retinopathies, scatter laser photocoagulation can be used to treat ischemic retina to induce regression of PRR.16 Management of complications due to PRR, including retinal detachment and vitreous hemorrhage, may require pars plana vitrectomy as definitive treatment. However, the need for surgery remains uncommon, with a retrospective study finding that vitrectomy was required in only 10 cases for vitreous hemorrhage, 5 cases for retinal detachment, and 1 case for combined vitreous hemorrhage/retinal detachment out of a total of 715 cases of radiotherapy used to treat posterior uveal melanoma.17
The use of hyperbaric oxygen therapy has also been investigated as a potential treatment for RR.18 Hyperbaric oxygen therapy involves the administration of oxygen at high pressures, which increases oxygen delivery to hypoxic tissues and may improve perfusion through damaged vessels. Also, pentoxifylline, a vasoactive agent used to treat claudication, has been reported to improve perfusion in RR by causing vasodilation and decreased blood viscosity.19
PREVENTION
Prevention of RR is a critical aspect of the management of patients undergoing radiation therapy for ocular and nonocular malignancies. Generally, the accepted radiation threshold is 3,000 rads (30 Gy) total delivered in equal doses across 3 weekly treatments, which each themselves are divided into 5 daily equal fractions of 200 rads.20 Radiation doses greater than this threshold may result in a greater occurrence and severity of RR, which warrants close monitoring.
Additional strategies have been proposed to reduce the risk of RR, including the use of proton therapy image-guided radiation therapy and dose fractionation. Image-guided radiation therapy uses real-time imaging techniques to precisely deliver radiation to a tumor while minimizing radiation exposure to surrounding healthy tissue. This approach can reduce the risk of RR by limiting the total amount of radiation delivered into the eye for treatment effect. Dose fractionation involves dividing the total radiation dose into smaller, more frequent doses. For example, in external beam radiation therapy, hyperfractionation allows the administration of a dose below 1.9 Gy/fraction, which can reduce the risk of RR by allowing the retina to recover between radiation treatments.
Specific to plaque radiotherapy for uveal melanoma, prophylactic treatment with intravitreal bevacizumab (Avastin; Genentech) injection every 4 months showed decreased rates of ME evident on optical coherence tomography, clinically evident maculopathy, and moderate vision loss over 2 years of follow-up.21 Further studies are necessary to evaluate the prophylactic role of other anti-VEGF agents and steroids to prevent RR.
To reduce the radiation dose for uveal melanoma patients with I-125 plaque brachytherapy, pars plana vitrectomy with silicone oil tamponade has also been attempted.22,23 The authors concluded that the silicone oil may provide a tissue-protective feature in patients with tumors distal to the macula. However, this technique has largely been abandoned due to the significantly higher incidence of metastases in the silicone oil–treated group. Thus, any potential benefits of RR prevention need to be weighed against the potential risk of metastatic spread.
FUTURE DIRECTIONS
The upcoming DRCR Protocol AL will evaluate intravitreal faricimab 6.0 mg injections (Vabysmo; Genentech) or intravitreal fluocinolone acetonide 0.19 mg implants vs observation to prevent acuity loss due to radiation retinopathy.24 Additional studies evaluating the use of newer, longer acting anti-VEGF and anti-angiopoietin agents, micropulse laser, and hyperbaric oxygen therapy are warranted. Prevention strategy studies are needed to evaluate the role of prophylactic therapy and optimal radiotherapy techniques to minimize the incidence of RR and its complications.
Radiation retinopathy is a progressive, sight-threatening complication of radiation therapy that occurs due to damage to the retinal vasculature. The severity and onset of RR depend on several factors, and its clinical presentation can be variable. Prevention of RR is critical, and image-guided radiation therapy and dose fractionation have been proposed to reduce the risk of RR. Management of RR is aimed at preventing further visual loss, with treatment options including anti-VEGF therapy, corticosteroids, laser photocoagulation, and more. RP
REFERENCES
- Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy--clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond). 1991;5(Pt 2):239-251. doi:10.1038/eye.1991.39
- Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(4):765-773. doi:10.1016/0360-3016(94)90347-6
- Packer S, Rotman M. Radiotherapy of choroidal melanoma with iodine 125. Int Ophthalmol Clin. 1980;20(2):135-142.
- Giuliari GP, Sadaka A, Hinkle DM, Simpson ER. Current treatments for radiation retinopathy. Acta Oncol. 2011;50(1):6-13. doi:10.3109/0284186X.2010.500299
- Bianciotto C, Shields CL, Pirondini C, Mashayekhi A, Furuta M, Shields JA. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117(5):1005-1012. doi:10.1016/j.ophtha.2009.10.015
- Kinyoun JL. Proliferative radiation retinopathy. Arch Ophthalmol. 1996;114(9):1097. doi:10.1001/archopht.1996.01100140299007
- Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:325-335.
- Kinyoun JL, Zamber RW, Lawrence BS, Barlow WE, Arnold AM. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol. 1995;79(2):144-149. doi:10.1136/bjo.79.2.144
- Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105(8):1425-1429. doi:10.1016/S0161-6420(98)98023-X
- Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2016;26(1):60-66. doi:10.5301/ejo.5000670
- Seibel I, Vollhardt D, Riechardt AI, et al. Influence of ranibizumab versus laser photocoagulation on radiation retinopathy (RadiRet) - a prospective randomized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):869-878. doi:10.1007/s00417-020-04618-7
- Murray TG, Latiff A, Villegas VM, Gold AS. Aflibercept for radiation maculopathy study: a prospective, randomized clinical study. Ophthalmol Retina. 2019;3(7):561-566. doi:10.1016/j.oret.2019.02.009
- Yu HJ, Fuller D, Anand R, et al. Two-year results for ranibizumab for radiation retinopathy (RRR): a randomized, prospective trial. Graefes Arch Clin Exp Ophthalmol. 2022;260(1):47-54. doi:10.1007/s00417-021-05281-2
- Russo A, Reibaldi M, Avitabile T, et al. Dexamethasone intravitreal implant vs ranibizumab in the treatment of macular edema secondary to brachytherapy for choroidal melanoma. Retina. 2018;38(4):788-794. doi:10.1097/IAE.0000000000001585
- Fallico M, Chronopoulos A, Schutz JS, Reibaldi M. Treatment of radiation maculopathy and radiation-induced macular edema: a systematic review. Surv Ophthalmol. 2021;66(3):441-460. doi:10.1016/j.survophthal.2020.08.007
- Augsburger JJ, Roth SE, Magargal LE, Shields JA. Panretinal photocoagulation for radiation-induced ocular ischemia. Ophthalmic Surg. 1987;18(8):589-593.
- Chia SN, Smith HB, Hammer HM, Kemp EG. Incidence and indications for pars plana vitrectomy following the treatment of posterior uveal melanomas in Scotland. Eye (Lond). 2015;29(6):748-756. doi:10.1038/eye.2015.20
- Gall N, Leiba H, Handzel R, Pe’er J. Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye (Lond). 2007;21(7):1010-1012. doi:10.1038/sj.eye.6702820
- Gupta P, Meisenberg B, Amin P, Pomeranz HD. Radiation retinopathy: the role of pentoxifylline. Retina. 2001;21(5):545-547. doi:10.1097/00006982-200110000-00026
- García-O’Farrill N, Pugazhendhi S, Karth PA, & Hunter AA. Radiation retinopathy intricacies and advances in management. Semin Ophthalmol. 2022;37(4):417-435. doi: 10.1080/08820538.2021.2000623
- Shah SU, Shields CL, Bianciotto CG, et al. Intravitreal bevacizumab at 4-month intervals for prevention of macular edema after plaque radiotherapy of uveal melanoma. Ophthalmology. 2014;121(1):269-275. doi:10.1016/j.ophtha.2013.08.039
- McCannel TA, Kim E, Kamrava M, et al. New ultra–wide-field angiographic grading scheme for radiation retinopathy after iodine-125 brachytherapy for uveal melanoma. Retina. 2018;38(12):2415-2421. doi:10.1097/IAE.0000000000001874
- Lyons LJ, Hinds ED, Chexal S, Berger B. Silicone oil and iodine-125 brachytherapy for uveal melanoma in high-risk patients. Cureus. 2019 Jul 29;11(7):e5270. doi:10.7759/cureus.5270.
- Jaeb Center for Health Research. Protocols in development. Accessed April 11, 2023. https://public.jaeb.org/drcrnet/view/UpcomeStudies








