Since corticosteroids were discovered in the 1950s, they have become and remain the mainstay of treatment for uveitis.1 Although the full mechanism of corticosteroids is not understood, it is believed that the basis for the anti-inflammatory and immunosuppressive effects of corticosteroids in the eye is the activation of the glucocorticoid receptor. The complete pathway is complex, but several important downstream effects include inhibition of neutrophil migration, interference with lymphocyte activity, inhibition of phospholipase A2, and a decrease in capillary permeability and fibroblast proliferation.2,3
Because uveitis affects 52 to 341 per 100,000 people per year in the United States,4,5 a firm understanding of the array and nuance of the various types of corticosteroids is an essential tool in the ophthalmologist’s toolkit.
TOPICAL STEROIDS
Topical corticosteroids are used primarily to treat inflammation in the anterior segment, due to poor penetration of the posterior segment. Their main advantage is the ability to achieve therapeutic concentrations in the anterior chamber with ease of administration and limited systemic side effects, especially when compared to systemically administered corticosteroids.
Several variables dictate the efficacy of topical corticosteroids in uveitis. Potency of various preparations of topical corticosteroids depend on their concentration, ability to penetrate the corneal epithelium, and durability in the anterior chamber (or vitreous). These factors favor molecules with an acetate such as prednisolone acetate and difluprednate.2 Furthermore, the 2 fluorinations of difluprednate 0.05% make dosing 4 times a day as potent as 8 times a day of prednisolone acetate 1% in the control of inflammation.6-8 Other structural changes, such as the substituted ester molecule in loteprednol, lead to rapid inactivation.9 Dosing frequency has been shown to change the anti-inflammatory effect of topical corticosteroids; Leibowitz showed, in a rabbit study, a 5-fold greater decrease in anterior-chamber neutrophils when prednisolone acetate 1% was dosed hourly compared to every 4 hours.10
Increased dosing frequency has led to several paradigms of topical steroid treatments, which can be summarized as: “hit it hard, hit it fast,” or more subtly, “use enough, soon enough.” Both phrases suggest that the maximum safe dose of steroid should be used early. Because aggressive, early steroid use can limit the overall time course of steroids, it may limit the total steroid burden and decrease risk of side effects such as increased intraocular pressure (IOP) or cataract.11,12 This is especially true for robust acute anterior segment inflammatory reactions.
In chronic inflammatory conditions of the anterior segment, topical corticosteroids can be used in conjunction with immunomodulatory therapy, especially if the treatment burden is thought to put the patient at low risk for steroid-related ocular adverse effects. Further, steroid monotherapy can and should be considered, especially if there is a low perceived risk of these side effects (ocular hypertension [OHTN], cataract), for example, if the disease process is easily controlled with a single drop of prednisolone acetate 1% dosed daily or every other day. Ocular hypertension occurs in about one third of patients on topical corticosteroids. It usually occurs after 4 to 6 weeks but may occur any time, usually after at least 5 days.12-14
SYSTEMIC CORTICOSTEROIDS
Oral corticosteroids play an important role in noninfectious posterior segment uveitis (intermediate, posterior, and panuveitis), retinal vasculitis, papillitis, and orbital inflammation. They are particularly useful in patients who have systemic manifestations of their ocular disease. However, oral corticosteroids can also be of value in the treatment of anterior uveitis unresponsive or only partially responsive to topical corticosteroids, as can be seen in HLA-B27–associated acute anterior uveitis.
Dosing for oral corticosteroids is dependent on the patient’s clinical presentation and course, but many physicians will start prednisone, a common oral steroid, dosed at 0.5 mg/kg to 1.5 mg/kg daily.15 Generally speaking, an initial dose of 20 mg to 40 mg of prednisone per day may be reasonable for mild inflammation; 40 mg to 60 mg per day might be considered for severe inflammation; doses as high as 80 mg to 100 mg daily (or more) may be necessary for resistant inflammation 48 hours after the initial dose.2 Equivalent doses of other oral steroids may be used.
After the inflammation is controlled, oral corticosteroids should ideally be tapered and maintained to prevent rebound inflammation. The typical goal is to decrease the daily dose to the lowest that will keep the inflammation at bay. Data from studies in rheumatoid arthritis show that prednisone doses less than 7.5 mg are associated with significantly lower rates of systemic side effects.16,17 At times, the addition of or transition to a biologic drug may facilitate long-term disease control while minimizing long-term steroid effects. However, if the chronic disease process being treated is adequately controlled at daily prednisone doses less than or equal to 7.5 mg, steroids alone may be a reasonable treatment option.
Intravenous corticosteroids play a role in immediate vision-threatening diseases. A common choice, methylprednisolone sodium succinate, may be pulse dosed at 1,000 mg per day for 3 days before transitioning to oral options.
Systemic corticosteroid side effects are extensive and exhaustedly studied. They include adrenal insufficiency, Cushing syndrome, peptic ulceration, osteoporosis, diabetes, infection, mood changes, and weight gain. Comanagement with primary care and rheumatology may be necessary in long-term steroid use, especially if the inflammatory condition cannot be adequately controlled with daily doses of prednisone of 7.5 mg or less.
PERIOCULAR CORTICOSTEROID INJECTIONS
Periocular steroids, whether by subconjunctival, sub-Tenon capsule, trans-septal, or retrobulbar injection, were previously used frequently in cases of severe or recalcitrant inflammation. In 2018, Thorne et al published the results of the POINT trial, which studied the treatment of uveitic macular edema by comparing periocular triamcinolone acetonide, intravitreal triamcinolone acetonide, and the intravitreal dexamethasone implant.18 The authors found that all modalities helped to improve macular edema, but the 2 intravitreal forms of steroid were superior in terms of reduction in the central subfield thickness on optical coherence tomography 8 weeks after treatment. These groups also had a greater percentage of patients who had resolution of macular edema at weeks 4 through 12 of the study period.
Once infectious etiologies have effectively been ruled out, periocular corticosteroid injections may be a consideration in cases of anterior-segment and/or posterior-segment inflammation recalcitrant to topical and/or oral corticosteroids, especially in individuals in whom there is a desire to avoid systemic steroid-related effects. The therapeutic effects typically last 2 to 3 months.19
Ocular hypertension is common in patients on periocular steroids. One study found a 5 mmHg increase in IOP in 44% of patients with peak incidence at 6 weeks.20 Cataract formation within 1 year occurs in 21% of patients.21 Local steroid injections may also have severe complications associated with the procedure, which include ptosis, retinal detachment, optic nerve atrophy, and formation of preretinal membrane.2 Due to these factors, the fact that the injections can be uncomfortable, and the results of the POINT trial, the use of periocular steroids may be considered less favorable than the use of corticosteroids administered via alternative routes (intravitreal, suprachoroidal).
INTRAVITREAL CORTICOSTEROIDS
Intravitreal corticosteroids are powerful tools in uveitis due to their direct administration into the vitreous cavity. They can be used to treat noninfectious posterior segment uveitis and associated macular edema.
Triamcinolone acetonide is a suspension that is injected directly into the vitreous cavity. It has the advantage of being readily accessible, but lasts only 6 to 8 weeks, and may result in a “snow globe effect,” leaving the patient temporarily blinded.22 The duration of effect may be further shortened if the injected eye has previously undergone pars plana vitrectomy.
The intravitreal 0.7 mg dexamethasone implant (Ozurdex; Allergan/AbbVie) (Figure 1) is a biodegradable pellet that represents a durable option, typically lasting 3 to 6 months. It is contraindicated in patients who have a unicameral eye due to potential implant migration into the anterior segment, which can lead rapidly to corneal decompensation. In a prospective, randomized, sham-controlled trial, the 0.7 mg dexamethasone implant resolved vitreous haze in 47% of eyes compared to 12% in sham eyes. This effect persisted for 6 months.23

For a longer lasting intravitreal injection, the intravitreal 0.18 mg fluocinolone acetonide implant (Yutiq; Alimera Sciences) (Figure 2) is found to last 36 months.24 This option is best for pseudophakic patients, because clinical trials showed progression of cataracts in 56% of phakic patients.25
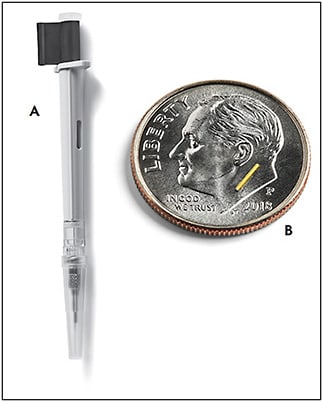
A surgically implanted intravitreal device, the 0.59 mg fluocinolone acetonide implant (Retisert; Bausch + Lomb) (Figure 3) can deliver corticosteroid for up to 3 years. In trials, it reduced the rate of recurrence of posterior uveitis from an untreated rate of 62% to a treated rate of 4% over 1 year and 20% over 3 years.26 The implantation involves creating a 3-mm to 4-mm incision in the sclera and suturing the device inside of the eye, which both is a barrier to administration and adds additional risk.

.
In all 3 intravitreal steroid implant options, OHTN and cataract are major adverse effects. Trials for the 0.7 mg dexamethasone implant found a rate of OHTN to be 7.1% and rate of cataract formation to be 15% over 6 months.23 For the 0.18 mg fluocinolone acetonide implant, 43% of patients required IOP lowering medication, and 53% of patients had progression of cataract.25 Trials of the 0.59 mg fluocinolone acetonide implant found rates of OHTN to be 67%, with 73% requiring IOP lowering drops and 40% requiring glaucoma filtering surgery. During the 3-year study, 93% of patients underwent cataract surgery.27 It is important to note that these rates of OHTN may underpredict real-world rates, because some trials excluded patients with a history of OHTN from previous steroid treatments.
There have been no prospective head-to-head comparisons of the 0.7 mg dexamethasone, the 0.18 fluocinolone acetonide, or the 0.59 mg fluocinolone acetonide implants in the treatment of uveitis. A retrospective study compared 0.7 mg dexamethasone and 0.59 mg fluocinolone acetonide implants and found no statistically significant difference in efficacy, but a greater rate of cataract and glaucoma in the fluocinolone group.28 Another retrospective study in patients with diabetic macular edema who transitioned from the 0.7 mg dexamethasone implant to the 0.18 mg fluocinolone acetonide implant showed improvement in visual acuity and central subfield thickness on optical coherence tomography.29
In addition to only lasting 3 months, the 0.7 mg dexamethasone implant is FDA approved for more indications (diabetic macular edema and macular edema after vein occlusion) and thus may be more readily available; it is therefore a reasonable first choice in intravitreal options. If the efficacy of intravitreal corticosteroid is proven, the patient can be committed to longer acting options.
CHOOSING BETWEEN INTRAVITREAL AND SYSTEMIC CORTICOSTEROIDS
The MUST trial was a randomized, double-blinded study comparing systemic therapy (including immunomodulatory agents) to the 0.59 mg fluocinolone acetonide implant. It found that, at 54 months, best corrected visual acuities were not statistically different between the 2 groups; however, there was improved control of inflammation at all time points in the intravitreal steroid group. The authors concluded that systemic corticosteroids are a cost-effective, reasonable initial option in early or bilateral intermediate, posterior, or panuveitis, but that intravitreal corticosteroid implants provide consistently better control of inflammation.27
This sentiment was further corroborated by a recent Cochrane Library systematic review compiling all randomized clinical trials comparing corticosteroid implants and standard-of-care therapy (systemic corticosteroids, intravitreal steroids, or disease-modifying therapies) or sham therapies for uveitis. The authors found that intravitreal corticosteroid implants reduced risk of recurrence compared to standard-of-care therapy by 54%.30
SUPRACHOROIDAL CORTICOSTEROIDS
Injections of corticosteroids into the suprachoroidal space promises to be a more targeted treatment for uveitic macular edema. The randomized, double-masked PEACHTREE trial highlighted the efficacy and safety of the 4 mg triamcinolone acetonide suprachoroidal injection (Xipere; Clearside Biomedical) (Figure 4). In this trial, 47% had a 15-letter improvement in visual acuity compared to 15% in the sham group during the 6-month trial. One of the most notable findings was a 7.3% rate of OHTN requiring treatment.31 Although a direct comparison with other trials’ rates of OHTN is not appropriate, the theoretical unique compartmentalization of steroid medication to the suprachoroidal space may limit its effect on the anterior segment and subsequent IOP changes.

Drug delivery via the suprachoroidal space provides another durable weapon in the armamentarium in the fight against posterior segment uveitis. And, unlike with some of the other previously mentioned injectable corticosteroid products, suprachoroidal triamcinolone can be used safely even in the setting of an open or ruptured posterior capsule or aphakia.
CONCLUSION
Any and all of these types of injectable steroids can be used as monotherapy, especially if a patient has a difficult time tolerating systemic corticosteroids and/or immunosuppression. However, these steroid-based treatments, like oral corticosteroids, can also be utilized as “bridge” therapy to temporarily keep an inflammatory process suppressed while waiting for the effects of other systemic immunomodulatory therapeutic agents to begin working, or to address flares of previously suppressed chronic inflammatory disease. Lastly, any of these agents can be used to address persistent posterior-segment inflammation and/or macular edema when only partial control of the disease process is attained through immunosuppression. RP
REFERENCES
- Steffensen EH. Corticotropin, cortisone, and hydrocortisone in treatment of ocular disease. J Am Med Assoc. 1952;150(17):1660-1664. doi:10.1001/jama.1952.03680170014004
- Bartlett JD, Jaanus SD, eds. Clinical Ocular Pharmacology. 5th ed. Butterworth-Heinemann/Elsevier; 2008.
- Sulaiman RS, Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in the eye. Steroids. 2018;133:60-66. doi:10.1016/j.steroids.2017.11.002
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491-500; discussion 500. doi:10.1016/j.ophtha.2003.06.014
- Reeves SW, Sloan FA, Lee PP, Jaffe GJ. Uveitis in the elderly: epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology. 2006;113(2):307.e1. doi:10.1016/j.ophtha.2005.10.008
- Foster CS, Davanzo R, Flynn TE, McLeod K, Vogel R, Crockett RS. Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010;26(5):475-483. doi:10.1089/jop.2010.0059
- Sheppard JD, Toyos MM, Kempen JH, Kaur P, Foster CS. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase iii, multicenter, randomized study. Invest Ophthalmol Vis Sci. 2014;55(5):2993-3002. doi:10.1167/iovs.13-12660
- Wilson ME, O’Halloran H, VanderVeen D, et al. Difluprednate versus prednisolone acetate for inflammation following cataract surgery in pediatric patients: a randomized safety and efficacy study. Eye (Lond). 2016;30(9):1187-1194. doi:10.1038/eye.2016.132
- Valdes LM, Sobrin L. Uveitis therapy: the corticosteroid options. Drugs. 2020;80(8):765-773. doi:10.1007/s40265-020-01314-y
- Leibowitz HM, Kupferman A. Optimal frequency of topical prednisolone administration. Arch Ophthalmol. 1979;97(11):2154-2156. doi:10.1001/archopht.1979.01020020472014
- Friedman NJ, Kaiser PK. Essentials of Ophthalmology. 2007; Saunders.
- Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20(4):407-416. doi:10.1038/sj.eye.6701895
- Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics: I. the effect of dexamethasone* in the normal eye. Arch Ophthalmol. 1963;70(4):482-491. doi:10.1001/archopht.1963.00960050484010
- Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. the effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70:492-499. doi:10.1001/archopht.1963.00960050494011
- Lustig MJ, Cunningham ET. Use of immunosuppressive agents in uveitis. Curr Opin Ophthalmol. 2003;14(6):399-412. doi:10.1097/00055735-200312000-00014
- Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119-1124. doi:10.1136/ard.2008.092163
- Palmowski A, Nielsen SM, Boyadzhieva Z, et al. Safety and efficacy associated with long-term low dose glucocorticoids in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). doi:10.1093/rheumatology/kead088
- Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the periocular vs. intravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283-295. doi:10.1016/j.ophtha.2018.08.021
- Tanner V, Kanski JJ, Frith PA. Posterior sub-Tenon’s triamcinolone injections in the treatment of uveitis. Eye. 1998;12(4):679-685. doi:10.1038/eye.1998.168
- Jea SY, Byon IS, Oum BS. Triamcinolone-induced intraocular pressure elevation: intravitreal injection for macular edema and posterior subtenon injection for uveitis. Korean J Ophthalmol. 2006;20(2):99-103. doi:10.3341/kjo.2006.20.2.99
- Sen HN, Vitale S, Gangaputra SS, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121(11):2275-2286. doi:10.1016/j.ophtha.2014.05.021
- Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: the past, the present, and the future. Surv Ophthalmol. 2008;53(2):139-149. doi:10.1016/j.survophthal.2007.12.005
- Lowder C, Belfort R, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545-553. doi:10.1001/archophthalmol.2010.339
- Jaffe GJ, Foster CS, Pavesio CE, Paggiarino DA, Riedel GE. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve-month results. Ophthalmology. 2019;126(4):601-610. doi:10.1016/j.ophtha.2018.10.033
- EyePoint Pharmaceuticals, Inc. Yutiq [Package Insert]. EyePoint Pharmaceuticals, Inc; 2022. Accessed March 9, 2023. https://yutiq.com/downloads/YUTIQ-US-PI-022022.pdf
- Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191-1201. doi:10.1001/archopht.126.9.1191
- Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, et al. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: fifty-four-month results of the multicenter uveitis steroid treatment (MUST) trial and follow-up study. Ophthalmology. 2015;122(10):1967-1975. doi:10.1016/j.ophtha.2015.06.042
- Arcinue CA, Cerón OM, Foster CS. A Comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013;29(5):501-507. doi:10.1089/jop.2012.0180
- Vaz-Pereira S, Castro-de-Sousa JP, Martins D, et al. The outcomes of switching from short- to long-term intravitreal corticosteroid implant therapy in patients with diabetic macular edema. Ophthalmic Res. 2020;63(2):114-121. doi:10.1159/000503036
- Reddy A, Liu SH, Brady CJ, Sieving PC, Palestine AG. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst Rev. 2023;(1). doi:10.1002/14651858.CD010469.pub3
- Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948-955. doi:10.1016/j.ophtha.2020.01.006








