Each human cell has a copy of that person’s genome, which is made of 3 billion base pairs distributed on 23 chromosomes and approximately 20,000 genes. Many cells, but especially cancer cells (and cells traumatized as in an accident), leak a very small portion of their nuclear DNA outside of the cell, and because it is outside the cell it is called cell-free DNA (cfDNA). Cell-free DNA has been found in saliva, stool, ascites, pleural fluid, urine, aqueous, and blood. In blood, the cfDNA is in the plasma. During pregnancy, more than 10% of the DNA in a mother’s blood is from the fetus. In cancer cells, the cfDNA is constantly being shed. The tiny fragments of DNA extruded from cancer cells are only 50 to 150 base pairs — less than 0.00000005% of the cell’s DNA.
A TEST FOR CELL-FREE DNA
At Memorial Sloan Kettering Cancer Center (MSKCC), scientists sequenced more than 25,000 cancers to identify the most common driver molecular alterations. That generated a list of 826 exons of 129 genes that were the most common oncogenic mutations. It also targeted 560 common SNPs (single-nucleotide polymorphisms) and 40 introns involved in rearrangements. Simultaneously, the test, called MSK-ACCESS (Analysis of Circulating cfDNA to Evaluate Somatic Status), interrogates the buffy coat. It then subtracts the plasma results from the buffy coat. This provides information about whether a defect that is found in plasma is germline or if it is a result of clonal hematopoiesis. It filters our more than 10,000 germline and clonal hematopoiesis variants. The test detects SNPs at a level of 0.1%, insertions and deletions at 0.1%, and copy number alterations fold change of -1.5. Figure 1 is a list of the genes identified in the test. For retinoblastoma, all 27 exons are included in the analysis.
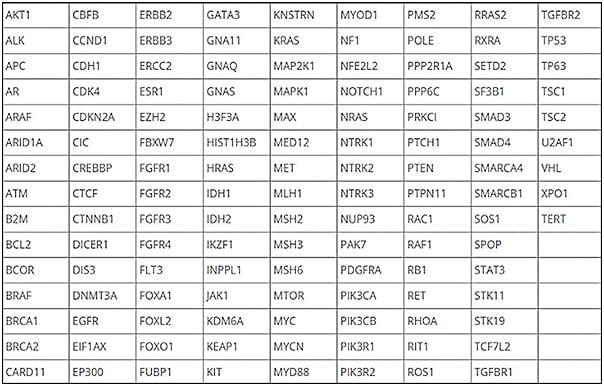
The results are expressed as variant allele frequency, which is the percentage of cfDNA present in the plasma. This was a research test at MSKCC from 2014 to 2019, and in 2019 it was approved by New York State as a clinical test for patients.1 The test has been performed more than 500 times in patients with retinoblastoma.
TESTING FOR CELL-FREE DNA PRIOR TO TREATMENT
In the initial report in 2020, we found RB1 cfDNA in 10 of 13 cases.2 A year later, we found it in 18 of 21 patients.1 Since then, we have expanded our analysis and the majority of all naïve retinoblastoma patients, unilateral or bilateral, have cell-free RB1 DNA detectable in blood. Presently we do not know why some patients do not have measurable cfDNA in blood, but it does appear that the eyes with extensive seeding explain some of the discrepancy. Vitreous seeding does not seem to get into the circulation.
UNTREATED RETINOBLASTOMA
At MSKCC, 3 patients had active retinoblastoma but for financial, social, and personal reasons did not have any medical intervention for a month. In 1 case, the variant allele frequency went from 0.34% to 2.28%, an increase of 21-fold. In a second case, one exon went from 14.99% to 17.8% while the other exon of the same patient went from 12.62% to 15.2%, an increase of about 20%. In the third case, a patient with recurrent intraocular disease went from 0 to 0.12%. This suggests that increasing levels of plasma cfDNA reflect increasing activity and progression of intraocular disease.
PSEUDORETINOBLASTOMA
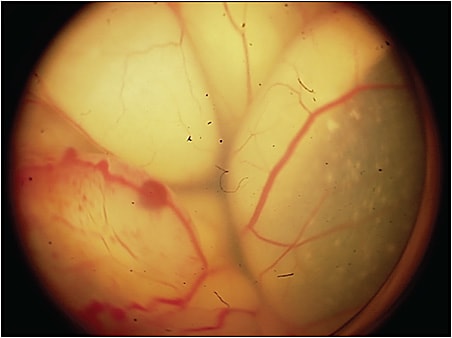
Unlike almost all human cancers, retinoblastoma is diagnosed without biopsy. Inadvertent entry into the eye of a child with retinoblastoma can be catastrophic; in a recent series, more than half of such patients developed metastatic disease and died.3 The differential diagnosis is extensive, with more than 30 intraocular lesions that simulate retinoblastoma. Some recent series show an error rate of 20% in some centers. Because of this, we have looked at a series of more than 30 typical retinoblastoma-simulating lesions, most common is Coats disease (Figure 2), and in all cases there was no RB1 cfDNA detected.4 A negative result does not mean the eye does not have retinoblastoma, as previously mentioned, but a positive result indicates that the lesion is not a simulating one.
OTHER SOMATIC MUTATIONS
The retinoblastoma gene was the first tumor suppressor gene cloned (almost 40 years ago) and is a textbook case of Mendelian autosomal dominant inheritance. As a result, the common assumption has been that the RB1 alteration is the only molecular derangement that occurs in retinoblastoma cells. Recent studies from labs around the world have shown, however, that not only are there additional non-RB1 (non-chromosome 13) mutations that develop, but also these are common and probably universal. There are many mutations that have been and continue to be identified, but a few happen more often than others. Although it is early in the story, it appears that some of these are associated with a worse outcome (ie, death). The MSK-ACCESS test of plasma in these patients has identified these mutations, which were formerly only detectable in enucleated specimens.4 Importantly, one of these aberrations is a BCL6 corepressor (BCOR) mutation.5 Patients whose tumors have BCOR mutations are more likely to develop metastases; plasma cfDNA has detected BCOR somatic mutations, and this may lead to different approaches (such as systemic chemotherapy) in these patients.
HALF-LIFE
Although it is known that the half-life of cfDNA is brief, most of the work has been done in animals. In humans, the half-life of a fetus’s cfDNA in the mother is 18 minutes after delivery of the placenta. Our group had the opportunity to do serial blood analyses of retinoblastoma patients during enucleation surgery; 50% of the cfDNA was gone in 10 minutes after cutting the optic nerve and 90% in 40 minutes, before the operation was even over.6
CELL-FREE DNA AFTER ENUCLEATION AND INTRA-ARTERIAL CHEMOTHERAPY
As mentioned above, plasma cfDNA rapidly disappears during enucleation surgery.7 It is never detectable after that. Our group has not measured patients every day after enucleation, but in >99% of patients, all cfDNA is gone 1 month after surgery. We have measured patients months, years, and decades later, and they are all negative.
The pattern following intra-arterial chemotherapy is similar.8 The majority of patients have no detectable plasma cfDNA when measured 1 month after intraarterial chemotherapy. By 3 months, almost all have no cfDNA. It never reappears if disease is controlled.
CELL-FREE DNA AND METASTASIS
This is an area of intense interest, and the full picture is not yet clear. In general, patients with the highest pretreatment levels of cfDNA are the ones who develop metastatic disease, but some eyes before treatment also have high levels of plasma cfDNA. Following treatment, if the cfDNA disappears, the children do not develop metastases, but if cfDNA is present after enucleation, clinical metastases develop within months. For patients with metastatic disease who have measurable cfDNA, levels disappear following successful treatment with high-dose multiagent systemic chemotherapy and transplant.
LEARNINGS FROM ANALYSIS OF THE BUFFY COAT
The buffy coat contains the white cells, so looking at it provides information about the germline molecular alterations. The plasma contains both pieces from the germline and somatic alterations but the test subtracts the buffy coat, so the reported results in the plasma are only from somatic alterations.
There are 2 important findings from analysis of the buffy coat:
- Mosaicism: Because of signal/noise issues, analysis of the buffy coat with MSK-ACCESS is hundreds of times more sensitive to detecting germline mosaicism. Very low level mosaicism (<5%) is often detected in patients who have had conventional, modern genetic testing and analysis. Because of this, it is possible that that mosaicism (very low) is very common.
- Detection of additional germline abnormalities, some that predispose to cancer: At MSKCC, extensive studies have demonstrated that children and adults with cancer harbor additional germline defects known to predispose patients to cancer in 10% to 17% of cases.9 In other words, they have cancer but it is probably unrelated to an additional germline defect, for example, a child with RB1 defect having a BRACA2 germline defect also. To date, 10% of patients with retinoblastoma also have a heritable germline predisposition to cancer. The implications of this are profound, as they may affect everything from a child’s diet, exposure to sun, screening, to even prophylactic surgery for gene-related cancers that have nothing to do with retinoblastoma.
CONLCUSION
A blood test for cancer, and in particular for retinoblastoma, is now a reality. The implications for patients with retinoblastoma and for clinicians managing their care could be far reaching. RP
REFERENCES
- Abramson DH, Mandelker D, Francis JH, et al. Retrospective evaluation of somatic alterations in cell-free DNA from blood in retinoblastoma. Ophthalmol Sci. 2021;1(1):100015. doi:10.1016/j.xops.2021.100015
- Kothari P, Marass F, Yang JL, et al. Cell-free DNA profiling in retinoblastoma patients with advanced intraocular disease: an MSKCC experience. Cancer Med. 2020;9(17):6093-6101. doi:10.1002/cam4.3144
- Kaliki S, Taneja S, Palkonda VAR. Inadvertent intraocular surgery in children with unsuspected retinoblastoma: a study of 14 cases. Retina. 2019;39(9):1794-1801. doi:10.1097/IAE.0000000000002214
- Francis JH, Richards AL, Mandelker DL, et al. Molecular changes in retinoblastoma beyond RB1: findings from next-generation sequencing. Cancers (Basel). 2021;13(1):149. doi:10.3390/cancers13010149
- Kooi IE, Mol BM, Massink MP, et al. Somatic genomic alterations in retinoblastoma beyond RB1 are rare and limited to copy number changes. Sci Rep. 2016;6:25264. doi:10.1038/srep25264
- Abramson DH, Mandelker DL, Brannon AR, et al. Mutant-RB1 circulating tumor DNA in the blood of unilateral retinoblastoma patients: what happens during enucleation surgery: a pilot study. PLoS One. 2023;18(2):e0271505. doi:10.1371/journal.pone.0271505
- Abramson DH. Cell free DNA (cfDNA) in the blood of retinoblastoma patients: the Robert M. Ellsworth lecture. Ophthalmic Genet. 2022;43(6):731-735. doi:10.1080/13816810.2021.2004433
- Francis JH, Gobin YP, Brannon AR, et al. RB1 circulating tumor DNA in the blood of patients with unilateral retinoblastoma: before and after intra-arterial chemotherapy. Ophthalmol Sci. 2021;1(3):100042. doi:10.1016/j.xops.2021.100042
- Fiala EM, Jayakumaran G, Mauguen A, et al. Prospective pan-cancer germline testing using MSK-IMPACT informs clinical translation in 751 patients with pediatric solid tumors. Nat Cancer. 2021;2:357-365. doi:10.1038/s43018-021-00172-1








