By 2050, 1 in 10 people on the planet will be highly myopic.1 High myopia is defined by the International Myopia Institute as a condition in which the spherical equivalent refractive error of an eye is less than or equal to -6.00 D when ocular accommodation is relaxed.2 This anticipated 4-fold increase in prevalence of high myopia since 2000 brings with it the many retina sequelae of increased axial elongation, including myopic traction maculopathy (MTM), myopic macular degeneration (MMD), and retinal detachments (RDs).3 The rise in myopic RDs alone uniquely challenges the retina surgeon with a far reach, tricky posterior vitreous detachment (PVD) inductions, and less efficacious chromovitrectomy against a backdrop of chorioretinal atrophy when peeling. Retina surgeons will find themselves increasingly in want of better tools and techniques to combat the downstream consequences of the myopia epidemic.
EPIDEMIOLOGY OF RETINAL DETACHMENTS IN HIGH MYOPIA
The incidence of RDs is increasing globally, with data from Denmark confirming a 50% increase in RDs over the last 16 years.4 A study conducted in Scotland among the general population revealed that the increase in RDs was mainly attributed to men aged 50 and above.5 The same study also found a strong association between myopia and early onset RDs, with myopia diagnosed in 82.1% of phakic RDs in individuals under the age of 50.5 There are a number of factors that influence the rise of RDs in myopes, including the younger age at onset of PVD,6,7 greater axial elongation leading to increased peripheral retinal thinning and peripheral retinal pathology,8 and the increased perpendicular and tangential tractional forces in the posterior pole of a staphylomatous eye resulting in myopic foveal detachments.9
In myopes, vitreous liquefaction occurs at an earlier age, resulting in earlier onset of PVDs.7 This occurs in accordance with level of myopia, with those with severe high myopia (<-10.00 D) developing a PVD earlier than those with moderate high myopia (-6.00 D to -10.00 D).6,10 This, coupled with the increased peripheral retinal thinning and prevalence of peripheral retinal pathology in myopes, results in a greater risk and earlier onset of RDs in high myopes.
As with PVDs, the lifetime risk of RD in myopia is proportional to the axial length and spherical equivalent (SE). The Eye Disease Case Control Study Group found that compared with emmetropes, eyes with an SE of -1.00 D to -3.00 D had a 4-fold increased risk of RD, and eyes with an SE of less than -3.00 D had a 10-fold increased risk of RD.11 Without surgery, the lifetime risk of RD in high myopia, as defined by Burton as less than -5.00 D, is 20-fold higher than in emmetropia.12
Ocular surgery may further compound this risk. Although the safety of cataract surgery has dramatically increased since its inception, the risk of RD after surgery for myopes is far greater than for emmetropes. Al Muammar et al found a 7-fold increase in RD in high myopes after cataract surgery as compared to emmetropes.13 In studying patients in the National Health Service in the United Kingdom, Williams et al found an overall incidence rate of 226.45 per 100,000 person-years after cataract extraction.14 Ultimately, the risk of RD after cataract surgery is again proportional to the axial length and SE going into the surgery.15
In addition to rhegmatogenous RDs, high myopes with staphylomatous eyes are also at risk for MTM that may progress to rarer, though more challenging to manage, macular holes with retinal detachment (MHRD) and foveoschisis retinal detachment (FSRD).16 Macular holes with retinal detachments occur specifically in high myopes with posterior staphylomas. The staphylomatous eye produces tangential traction of the retina when the preretinal vitreous cortex adheres to the retina around a macular hole.9 Given that this traction is difficult to release even with surgery, MHRDs in patients with high myopia have a lower closure rate compared to those with nonmyopic eyes. Similarly, FSRD without macular holes tend to occur in extreme cases of staphyloma and may precede MHRDs.17 Foveoschisis RDs occur secondary to tractional forces elongating the eye perpendicularly to the retinal plane.9
MANAGEMENT OF RETINAL DETACHMENTS IN HIGH MYOPIA
Preparation is critical for surgical interventions in high myopes. The most important preoperative step is to obtain an axial length measurement because this will inform the surgeon as to instruments needed. Unless a dense media opacity is present, optical biometry is more accurate, even in the case of an eye filled with silicone oil.18
Vitrectors and microforceps can vary both by supplier and by gauge. Alcon 23-gauge vitrectors measure 31.75 mm, whereas 25-gauge+ and 27-gauge+ have a working distance of 27 mm. Alcon 23-gauge microforceps measure 32 mm, 25-gauge measure 30 mm, and 25-gauge+ and 27-gauge+ measure 27 mm. DORC 23-gauge disposable microforceps range from 33 mm to 34.8 mm, with high myopic reusable forceps measuring 40 mm in effective reach. DORC 25-gauge disposable microforceps range from 29.9 mm to 31.2 mm, with high myopic reusable forceps measuring 35.7 mm in effective reach. Lastly, DORC 27-gauge disposable microforceps range from 25.5 mm to 29.1 mm in effective reach. With longer axial lengths, larger gauge surgery should be considered, and the surgeon should plan to suture sclerotomies at the case end to prevent hypotony, given the more pliable sclera in high myopes with posterior staphyloma.
Additionally, retina surgeons must perform a thorough preoperative scleral depressed exam (SDE) for tears and thorough intraoperative SDE for tears at the end of the case. High myopes tend to have more posterior vitreous base insertions that may result in more posterior tears (Figure 1). Any missed break could be detrimental to the success of the case.

MACULAR HOLE WITH RETINAL DETACHMENT
The unique cases of myopic MHRD and FSRD must be approached with even greater preoperative planning than the standard rhegmatogenous RD. Management of MHRD has fluctuated from primary macular buckling to pars plana vitrectomy (PPV)19 and now, at least internationally, back to macular buckling in combination with PPV vs either surgery alone (Figure 2). Gonvers and Machemer first reported successful treatment of 6 MHRDs in 1982 using PPV, partial gas-fluid exchange, and prone positioning for 12 hours.20 Of the 6 cases, 5 were in high myopes whose refractive error ranged from -7.75 D to -19 D. One MHRD recurred and was successfully treated with a second partial gas-fluid exchange and prone positioning.
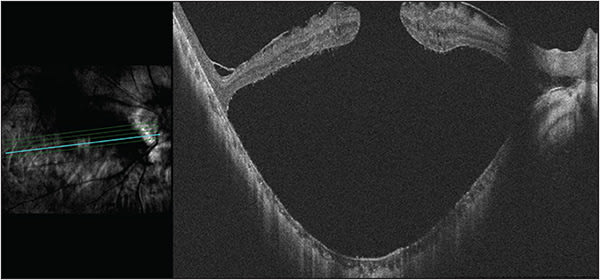
Since that time, internal limiting membrane (ILM) peeling with indocyanine green or brilliant blue G staining has been added to vitrectomy surgery to increase the success of peeling. The inverted ILM flap technique developed by Michalewska et al in 2010 has further improved anatomic and functional outcomes of myopic full-thickness macular holes (FTMH). Rather than completely peeling the ILM, a small remnant is retained at the margin of the FTMH. The modified temporal inverted ILM flap improved upon this technique by preserving the nasal ILM, decreasing the risk of dimpling of the fundus or dissociated optic nerve fiber layer appearance.21
Given overall lower reported anatomical success rates in highly myopic eyes with posterior staphyloma, interest in macular buckles has resurged.22 Macular buckling was first described by Schepens in 1957 when he used a short polyethylene tube sewn onto the sclera for macular breaks.23 It has since evolved with the development of a T-shaped silicone plate embedded with titanium wires for rigidity, called a macular plombe or “Ando’s plombe” after Dr. Fumitaka Ando who designed it.24 This and modified techniques including use of illuminated light pipes, adjustable buckles, and intraoperative optical coherence tomography (OCT) for placement may improve the safety of macular buckling.25,26 Alternative methods of posterior scleral reinforcement, including the use of suprachoroidal long-acting hyaluronic acid, have also since been described.27,28 However, in addition to the hyperopic shift that often results in diplopia, the procedure is risky given its target location. Complications that include macular subretinal hemorrhage, chorioretinal atrophy at the indentation area of the buckle, posterior pole retinal pigmented epithelium atrophy, posterior globe perforation, suprachoroidal hemorrhage, motility restriction, optic nerve abutment, and buckle extrusion have been reported.24 Still, macular buckling alone or in combination with PPV may have better postoperative anatomic and functional results compared with PPV in those with extreme myopia (>30 mm), pronounced posterior staphyloma, and posterior vitreous schisis.24,29-33 Careful preoperative planning with high-resolution OCT and B-scan ultrasonography can add to the accuracy of the procedure.
Unfortunately, to the authors’ knowledge, there are no commercially available exoplants in the United States. In Europe, 3 options exist: the AJL Macular Buckle (AJL Ophthalmic), the T-shaped Buckle (FCI Ophthalmics), and the Adjustable Macular Buckle (Micromed).
FOVEOSCHISIS WITH RETINAL DETACHMENT
Tractional forces elongating the eye perpendicularly to the retinal plane can result in foveoschisis with RD without breaks. Similar to MHRDs, FSRDs may be treated with PPV with or without ILM peeling34 and/or macular buckling (Figure 3). Again, cases with extreme myopia (>30 mm axial length) may be most likely to benefit from the inclusion of a macular buckle.

Unique to FSRDs, a “fovea-sparing” technique has been developed for the ILM peel to avoid the induction of FTMHs. The inflexibility of the ILM while the eye elongates is thought to contribute to the onset of foveoschisis, which is why ILM peeling is commonly performed along with PPV in such cases. Although ILM peeling may relieve tractional forces perpendicular to the retinal plane, it also increases the risk of postoperative development of a FTMH in high myopes. Therefore, Shimada et al developed the technique of fovea-sparing ILM peel to mitigate this risk.35 The ILM peel is limited to the parafovea and perifovea, eliminating tangential traction adjacent the fovea. Using this technique, Shimada et al found that 0 of 15 eyes developed FTMH as compared to 5 of 30 eyes in the complete ILM peel group.35
CONCLUSION
Retinal detachments in the setting of high myopia are increasing in prevalence around the world. Vitreoretinal surgeons must measure the axial length prior to surgery to ensure they have adequate reach and must perform a thorough preoperative and intraoperative exam to ensure no breaks are missed. Detachments in the setting of MTM, although historically rare, are likely to increase with the myopia epidemic. They are challenging to repair and may recur as the MTM progresses. Newer surgical techniques, as well as the modification of older techniques, may result in higher surgical success. RP
REFERENCES
- Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. doi:10.1016/j.ophtha.2016.01.006
- Flitcroft DI, He M, Jonas JB, et al. IMI – defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20. doi:10.1167/iovs.18-25957
- Cheung N, Lee SY, Wong TY. Will the myopia epidemic lead to a retinal detachment epidemic in the future? JAMA Ophthalmol. 2021;139(1):93-94. doi:10.1001/jamaophthalmol.2020.5112
- Nielsen BR, Alberti M, Bjerrum SS, Cour M. The incidence of rhegmatogenous retinal detachment is increasing. Acta Ophthalmologica. 2020;98(6):603-606. doi:10.1111/aos.14380
- Mitry D, Charteris DG, Yorston D, et al. The epidemiology and socioeconomic associations of retinal detachment in scotland: a two-year prospective population-based study. Invest Ophthalmol Vis Sci. 2010;51(10):4963. doi:10.1167/iovs.10-5400
- Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100(9):1384-1388. doi:10.1016/s0161-6420(93)31471-5
- Ludwig CA, Shields RA, Chen TA, et al. A novel classification of high myopia into anterior and posterior pathologic subtypes. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1847-1856. doi:10.1007/s00417-018-4071-0
- Pierro L, Camesaca F, Mischi M, Brancato R. Peripheral retinal changes and axial myopia. Retina. 1992;12(1):12-17. doi:10.1097/00006982-199212010-00003
- Parolini B, Arevalo JF, Hassan T, et al. International validation of myopic traction maculopathy staging system. Ophthalmic Surg Lasers Imaging Retina. 2023;54(3):153-157. doi:10.3928/23258160-20230217-01
- Hayashi K, Manabe SI, Hirata A, Yoshimura K. Posterior vitreous detachment in highly myopic patients. Invest Ophthalmol Vis Sci. 2020;61(4):33. doi:10.1167/iovs.61.4.33
- Risk factors for idiopathic rhegmatogenous retinal detachment. The Eye Disease Case-Control Study Group. Am J Epidemiol. 1993;137(7):749-757.
- Burton TC. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc. 1989;87:143-155; discussion 155-157.
- Al Muammar AR, Al-Harkan D, Al-Rashidy S, Al-Suliman S, Mousa A. Frequency of retinal detachment after cataract surgery in highly myopic patients. Saudi Med J. 2013;34(5):511-517.
- Williams MA, McGimpsey S, Abugreen S, et al. The incidence and rate of rhegmatogenous retinal detachment seven years after cataract surgery in patients with high myopia. Ulster Med J. 2009;78(2):99-104.
- Thylefors J, Jakobsson G, Zetterberg M, Sheikh R. Retinal detachment after cataract surgery: a population-based study. Acta Ophthalmol. 2022;100(8):e1595-e1599. doi:10.1111/aos.15142
- Cheong KX, Xu L, Ohno-Matsui K, Sabanayagam C, Saw SM, Hoang QV. An evidence-based review of the epidemiology of myopic traction maculopathy. Surv Ophthalmol. 2022;67(6):1603-1630. doi:10.1016/j.survophthal.2022.03.007
- Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128(4):472-476. doi:10.1016/s0002-9394(99)00186-5
- Rajurkar K, DaCruz R, Thakar M. Comparison of optical biometry and conventional acoustic biometry in the axial length measurement in silicone oil-filled eyes. Indian J Ophthalmol. 2022;70(8):2851-2854. doi:10.4103/ijo.IJO_3019_21
- Ikuno Y, Tano Y. Vitrectomy for macular holes associated with myopic foveoschisis. Am J Ophthalmol. 2006;141(4):774-776. doi:10.1016/j.ajo.2005.11.044
- Gonvers M, Machemer R. A new approach to treating retinal detachment with macular hole. Am J Ophthalmol. 1982;94(4):468-472. doi:10.1016/0002-9394(82)90240-9
- Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: a comparative study. Retina. 2015;35(9):1844. doi:10.1097/IAE.0000000000000555
- Ikuno Y, Sayanagi K, Oshima T, et al. Optical coherence tomographic findings of macular holes and retinal detachment after vitrectomy in highly myopic eyes. Am J Ophthalmol. 2003;136(3):477-481. doi:10.1016/s0002-9394(03)00269-1
- Schepens CL, Okamura ID, Brockhurst RJ. The scleral buckling procedures. I. Surgical techniques and management. AMA Arch Ophthalmol. 1957;58(6):797-811. doi:10.1001/archopht.1957.00940010819003
- Susvar P, Sood G. Current concepts of macular buckle in myopic traction maculopathy. Indian J Ophthalmol. 2018;66(12):1772-1784. doi:10.4103/ijo.IJO_1126_18
- Forlini M, Szkaradek M, Rejdak R, et al. Modification of adjustable macular buckling with 29-g chandelier light for optimal positioning in highly myopic eyes with macular hole. Retin Cases Brief Rep. 2017;11(3):249-254. doi:10.1097/ICB.0000000000000361
- Bedda AM, Abdel Hadi AM, Abd Al Shafy MS. A comparative study between vitrectomy with internal tamponade and a new modified fiber optic illuminated Ando plombe for cases of macular hole retinal detachment in myopic eyes. J Ophthalmol. 2015;2015:841925. doi:10.1155/2015/841925
- Qi Y, Duan AL, You QS, Jonas JB, Wang N. Posterior scleral reinforcement and vitrectomy for myopic foveoschisis in extreme myopia. Retina. 2015;35(2):351-357. doi:10.1097/IAE.0000000000000313
- El Rayes EN. Suprachoroidal buckling in managing myopic vitreoretinal interface disorders: 1-year data. Retina. 2014;34(1):129-135. doi:10.1097/IAE.0b013e31828fcb77
- Ma J, Li H, Ding X, Tanumiharjo S, Lu L. Effectiveness of combined macular buckle under direct vision and vitrectomy with ILM peeling in refractory macular hole retinal detachment with extreme high axial myopia: a 24-month comparative study. Br J Ophthalmol. 2017;101(10):1386-1394. doi:10.1136/bjophthalmol-2016-310123
- Ripandelli G, Coppé AM, Fedeli R, Parisi V, D’Amico DJ, Stirpe M. Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: a comparison [corrected] of vitrectomy versus posterior episcleral buckling surgery. Ophthalmology. 2001;108(12):2258-2264; discussion 2265. doi:10.1016/s0161-6420(01)00861-2
- Parolini B, Frisina R, Pinackatt S, et al. Indications and results of a new l-shaped macular buckle to support a posterior staphyloma in high myopia. Retina. 2015;35(12):2469-2482. doi:10.1097/IAE.0000000000000613
- Mortada HA. A novel episcleral macular buckling: wire-strengthened sponge exoplant for recurrent macular hole and retinal detachment in high myopic eyes. Med Hypothesis Discov Innov Ophthalmol. 2013;2(1):14-19.
- Mura M, Iannetta D, Buschini E, de Smet MD. T-shaped macular buckling combined with 25g pars plana vitrectomy for macular hole, macular schisis, and macular detachment in highly myopic eyes. Br J Ophthalmol. 2017;101(3):383-388. doi:10.1136/bjophthalmol-2015-308124
- Ikuno Y, Sayanagi K, Ohji M, et al. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004;137(4):719-724. doi:10.1016/j.ajo.2003.10.019
- Shimada N, Sugamoto Y, Ogawa M, Takase H, Ohno-Matsui K. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. Am J Ophthalmol. 2012;154(4):693-701. doi:10.1016/j.ajo.2012.04.013








