Diabetic retinopathy (DR) is the leading cause of blindness globally and is the most common microvascular complication in patients with diabetes.1 It is well documented that DR development is strongly correlated with a longer duration of diabetes and suboptimal blood sugar and blood pressure control. However, an initial early worsening of DR has been reported in patients that receive treatment to rapidly normalize glycemic levels. This phenomenon has been described with therapies such as insulin and sulfonylureas.
In recent years, second-line noninsulin therapies such as glucagon-like peptide 1 receptor agonists (GLP-1RAs) have gained popularity as effective agents to sustainably alter blood glucose levels while exerting additional positive systemic effects. Despite this efficacy, some studies have revealed an increased risk of DR adverse events among patients treated with GLP-1RAs.2 Currently, the effects of GLP-1RAs on DR incidence, prevalence, and progression are not well understood or described in the ophthalmic literature. This article will explore the phenomenon of early worsening of diabetic retinopathy (EWDR), ocular concerns surrounding GLP-1RA, and implications of GLP-1RA use for systemic diabetic treatment regimens for the ophthalmic provider.
A PRIMER ON EARLY WORSENING OF DIABETIC RETINOPATHY
Early worsening of diabetic retinopathy (EWDR) is a phenomenon first described during intensive therapy (IT) in patients with type 1 diabetes (T1D) and subsequently in patients with type 2 diabetes (T2D). The term “early” refers specifically to the establishment of tighter glycemic control in patients and not the duration or onset of their diabetes.3 Early worsening of diabetic retinopathy is not an uncommon phenomenon; a systematic review of 19 publications found early worsening to arise in 10% to 20% of patients within 3 to 6 months after rapid improvements in glucose control.4 This condition is nearly 2 times more likely to occur in patients with advanced baseline DR. In addition to T1D and T2D, EWDR has also been observed in patients after bariatric surgery or pancreatic transplantation and in patients who are pregnant.
Early worsening often presents in patients who have long-term or uncontrolled diabetes and DR prior to beginning IT.4 Various systematic reviews and meta-analyses have specifically found larger magnitudes of reduction of HbA1c as probable indicators for the development of EWDR. Another potential risk factor for EWDR is suboptimal blood pressure control before a patient begins IT.
The definition of early worsening varies across trials examining this condition. Cumulatively, it is characterized by the appearance of cotton wool spots, soft exudates, retinal hemorrhages, microaneurysms, intraretinal microvascular abnormalities, or the progression of existing DR.5 Early worsening of diabetic retinopathy is often transient and is driven primarily by the development of cotton-wool spots and intraretinal microvascular abnormalities in patients with minimal to no DR. However, patients who have advanced DR before beginning IT can experience irreversible retinal damage. Currently, there is no consensus regarding the pathogenesis of this condition and there exist several theories currently outside the scope of this paper.
These potential complications may seem problematic for providers attempting to optimize their patient’s glycemic control and protect their visual potential. However, these transient findings are outweighed by the long-term benefits patients will experience. The landmark Diabetes Control and Complications Trial (DCCT) for patients with type 1 diabetes reported higher rates of EWDR in patients undergoing IT compared to the conventional treatment group.6 These patients tended to have higher baseline HbA1c levels and as a result, experienced larger reductions in their HbA1c in the first 6 months. However, despite this initial decline, after 12 months patients in the IT group experienced similar, if not favorable, retinopathy gradings compared to their conventional treatment counterparts. After 10 years of follow-up, the IT group had a significantly lower risk of DR progression, an effect that persisted for up to 18 years.7
This long-term benefit has been reflected by systematic reviews and meta-analyses examining both T1D and T2D. A meta-analysis of 24 studies involving 9,302 patients found intensive insulin therapy, specifically insulin pump therapy vs multiple daily injections, reduced DR progression by nearly 33% in both adults and adolescents with T1D.8 A 2014 Cochrane Review of 12 clinical trials and 2,230 participants with T1D found similar benefits (reduced risk of developing microvascular complications by 17%) in patients on IT.9 Finally, a meta-analysis of 4 studies of 27,049 participants with T2D found IT yielded a 13% risk reduction for ocular events.10
Although EWDR initially may be concerning to a provider, the long-term reduction of a patient’s risk of DR necessitates aggressive glycemic control to normalize blood sugar levels. These take-home points will be explored below in the drug-specific discussion of GLP-1.
EVIDENCE IN THE LITERATURE
GLP-1RAs are often subcutaneous injectable agents that work by activating GLP-1 receptors in the pancreas, leading to enhanced insulin release and reduced glucagon release. These agents have gained favor among clinicians to achieve glycemic control because of their additional positive effects on weight, beta cell function, cholesterol levels, and cardiovascular markers. Although EWDR was described as early as 1980, newer systemic agents such as GLP-1RAs have revealed mixed results regarding their correlation with DR and have been minimally explored in the ophthalmic literature.
There has been some exploration of EWDR in non-ophthalmic-focused publications. The first and most controversial recent clinical trial was titled “Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).”11 It suggested that semaglutide (a GLP-1RA) may be linked to increased adverse retinal events compared to placebo. The study found an increase of 76% in severe DR complications in the semaglutide group when compared to the placebo (P<.002). An additional clinical trial, LEADER,12 found higher (but not statistically significant) rates of adverse retinal events (P=.33). Subsequent cardiovascular clinical trials, such as EXSCEL,13 HARMONY,14 REWIND,15 and PIONEER-6,16 did not find a discernible difference in adverse events in patients treated with subcutaneous injectable GLP-1RA (except PIONEER-6, which used an oral formulation). These mixed results make it difficult to distinguish the relationship between EWDR and GLP-1 usage.
One study designed to examine this concern was the AngioSafe T2D study,17 which examined GLP-1RA’s role in angiogenesis in both clinical and experimental models. In a cohort of 3,154 patients with T2D, after conducting multivariate analysis, the study found sex, disease duration, HbA1c, microangiopathy and macroangiopathy, insulin therapy, and hypertension as strong correlates with severe DR. These are similar to the risk factors of general EWDR as described earlier. However, no statistically significant association was noted from GLP-1RA exposure (P=.47). Duration of exposure to GLP-1RA also did not significantly impact their analysis (P=.36). The experimental arm of the AngioSafe Type 2 Diabetes study further validated these results, finding no impact of exendin-4 (a GLP-1RA) on human endothelial cells or any negative effects on retinal neovascularization. Although useful, the retrospective cross-sectional nature of this investigation limits the extrapolation of its findings.
A meta-analysis18 was conducted in 2021 that incorporated 6 randomized GLP-1RA–specific Cardiovascular Outcome Trials (CVOTs), some of which are briefly discussed earlier. These studies provide long-term randomized, placebo-controlled follow-up for these agents, with a median follow-up of 3.4 years. Meta-analyses demonstrated no significant association between GLP-1RA and retinopathy risk (P=.290). This validates the findings of the AngioSafe T2D study. Instead, meta-regression in this trial revealed a significant association between the magnitude of HbA1c reduction and retinopathy. As mentioned earlier, this is an established and important risk factor in the development of EWDR. The SUSTAIN-6 trial, now considered an outlier among other CVOT trials, demonstrated the largest HbA1c differences recorded at various follow-up intervals, explaining much of the relationship between GLP-1RA exposure and DR adverse events. When specifically examining the SUSTAIN-6 trial, an A1c reduction as high as 2.5% was observed in the 1 mg semaglutide interventional group. Furthermore, SUSTAIN trials 1 through 5, along with a Japanese semaglutide cohort study, demonstrated no significant risk of DR adverse events.19
Although these investigations provide greater insight into systemic GLP-1 diabetic treatment and its impact on retinopathy, there are several limitations. These studies, although well designed, are mostly not sufficient to explore the impact of GLP-1RA on ocular outcomes. In many of these studies, retinal imaging methods and assessment of DR were inconsistent and lacking in external validation. This is also reflected in the heterogeneity statistic between studies (I2=52.2%; Q statistic P=.063) included within the meta-analyses.
Currently, the FOCUS clinical trial is recruiting participants to study the effects of semaglutide (which was reported to have the highest antihyperglycemic potential and described in the SUSTAIN-6 study) on specifically ocular complications. This study will last for 5 years, and evaluation of DR will be based on retinal imaging and the ETDRS protocol.20
BENEFIT-RISK RATIO
Intensive therapy offers significant benefits for the long-term frequency and progression of DR, a benefit which extends to patients utilizing GLP-1RAs. A meta-analysis conducted in 2017 compared 21,782 patients using GLP-1RAs against 17,296 patients using other systemic diabetes drugs. The investigators found that GLP-1RAs not only have no discernible impact on the frequency or progression of DR, but their subgroup analysis revealed GLP1-RA was associated with a lower risk of retinopathy in comparison with sulfonylureas.21
Interestingly, GLP-1RA has been reported to help maintain the integrity of the blood-retinal barrier and protect retinal cells and endothelium from hyperglycemic-induced damage.22 Recently, native GLP-1 and multiple GLP-1RA analogs have been explored as potential combatants against diabetes-induced neurodegeneration, neuroinflammation, and vascular leakage within retinal models.23,24 More specifically, their results suggest that GLP-1R activation may prevent retinal neurodegeneration. This potential area of study may provide additional routes to protecting against patients at risk from developing DR or advancing their current condition.
IMPLICATIONS FOR MANAGEMENT
Regardless of its transient status, EWDR has real connotations for clinical practice. It is important for ophthalmologists to be cognizant of their patient’s current systemic diabetic treatments. One paper25 recommends a comprehensive retinal exam to be performed prior to IT and for these patients to be closely followed over the next 12 months. In patients with mild to no DR, IT can be initiated safely with no ophthalmic intervention. However, in patients with severe nonproliferative or proliferative DR, anti-VEGF therapy and/or panretinal photocoagulation therapy should be considered. Finally, the authors recommend quarterly eye monitoring for patients at a high risk of EWDR (previous advanced DR or long-term uncontrolled diabetes) after a year of IT. In patients without these risk factors (short-term diabetes or minimal DR before IT), follow-up every 6 months should suffice. These recommendations have been summarized in Figure 1.
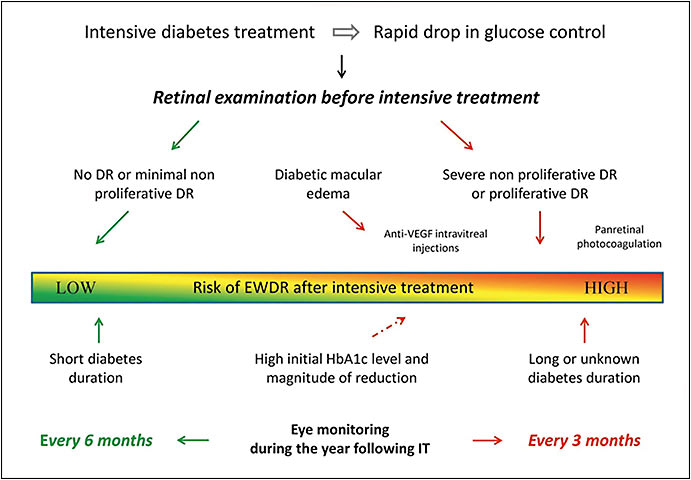
Finally, as part of a comprehensive treatment plan, it is important to emphasize lifestyle changes and proper dietary recommendations for these patients. A subgroup analysis of the DCCT trial found a high-calorie diet, especially rich in fatty acids, along with cigarette smoking were factors statistically significantly correlated with a higher risk of DR progression.26
CONCLUSION
Ophthalmic providers should be vigilant for signs of EWDR in their patients undergoing systemic glycemic treatment. Their decision to intervene is dependent on several factors, including the patient’s pre-existing level of DR and their baseline HbA1c. Existing meta-analyses and systematic reviews of GLP-1RAs have not demonstrated significant harmful effects of these agents on the development or progression of DR. Unfortunately, much of the existing literature regarding EWDR and GLP-1RAs is not significantly reflected in the ophthalmic literature. As a result, many questions remain and a rigorous methodology for DR assessment and evaluation of various diabetic agents must be encouraged to better elucidate this connection. RP
REFERENCES
- Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62(4):211-217. doi:10.1159/000499541
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
- Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes Obes Metab. 2019;21(3):454-466. doi:10.1111/dom.13538
- Feldman-Billard S, Larger É, Massin P. Standards for screening and surveillance of ocular complications in people with diabetes SFD study group. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metab. 2018;44(1):4-14. doi:10.1016/j.diabet.2017.10.014
- Dahl-Jørgensen K. Near-normoglycemia and late diabetic complications. The Oslo Study. Acta Endocrinol Suppl (Copenh). 1987;284:1-38.
- Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial [published correction appears in Arch Ophthalmol 1998 Nov;116(11):1469]. Arch Ophthalmol. 1998;116(7):874-886. doi:10.1001/archopht.116.7.874
- Akil H, Burgess J, Nevitt S, Harding SP, Alam U, Burgess P. Early worsening of retinopathy in type 1 and type 2 diabetes after rapid improvement in glycaemic control: a systematic review. Diabetes Ther. 2022;13(1):1-23. doi:10.1007/s13300-021-01190-z
- Virk SA, Donaghue KC, Wong TY, Craig ME. Interventions for diabetic retinopathy in type 1 diabetes: systematic review and meta-analysis. Am J Ophthalmol. 2015;160(5):1055-1064.e4. doi:10.1016/j.ajo.2015.07.024
- Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2014(2):CD009122. doi:10.1002/14651858.CD009122.pub2
- Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431-437. doi:10.1016/S2213-8587(17)30104-3
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-1239. doi:10.1056/NEJMoa1612917
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519-1529. doi:10.1016/S0140-6736(18)32261-X
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121-130. doi:10.1016/S0140-6736(19)31149-3
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841-851. doi:10.1056/NEJMoa1901118
- Gaborit B, Julla JB, Besbes S, et al. Glucagon-like peptide 1 receptor agonists, diabetic retinopathy and angiogenesis: the AngioSafe type 2 diabetes study. J Clin Endocrinol Metab. 2020;105(4):dgz069. doi:10.1210/clinem/dgz069
- Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC. HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: a meta-analysis and meta-regression. Diabetes Care. 2021;44(1):290-296. doi:10.2337/dc20-1815
- Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889-897. doi:10.1111/dom.13172
- A Research Study to Look at How Semaglutide Compared to Placebo Affects Diabetic Eye Disease in People With Type 2 Diabetes (FOCUS). ClinicalTrials.gov identifier: NCT03811561. Updated November 14, 2022. Accessed December 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03811561
- Dicembrini I, Nreu B, Scatena A, et al. Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized controlled trials [published correction appears in Acta Diabetol. 2017 Sept 19]. Acta Diabetol. 2017;54(10):933-941. doi:10.1007/s00592-017-1031-9
- Fan Y, Liu K, Wang Q, Ruan Y, Ye W, Zhang Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res. 2014;127:104-116. doi:10.1016/j.exer.2014.05.004
- Hernández C, Bogdanov P, Corraliza L, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2016;65(1):172-187. doi:10.2337/db15-0443
- Liu J, Wei L, Wang Z, et al. Protective effect of liraglutide on diabetic retinal neurodegeneration via inhibiting oxidative stress and endoplasmic reticulum stress. Neurochem Int. 2020;133:104624. doi:10.1016/j.neuint.2019.104624
- Feldman-Billard S, Larger É, Massin P; Standards for screening and surveillance of ocular complications in people with diabetes SFD study group. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metab. 2018;44(1):4-14. doi:10.1016/j.diabet.2017.10.014
- Cundiff DK, Nigg CR. Diet and diabetic retinopathy: insights from the Diabetes Control and Complications Trial (DCCT). MedGenMed. 2005;7(1):3.








