During the past 2 decades, the pace of research devoted to the development of retinal biomarkers for a range of neurodegenerative diseases1-3 and neurologic disorders4 has increased dramatically. Because most neurodegenerative diseases begin to develop years, or even decades, before the emergence of clinical symptoms, there are significant unmet needs for sensitive and reliable diagnostic tools capable of identifying the earliest stages of disease — a window of time during which novel or existing interventions could possibly succeed in halting or slowing disease progression.
Among promising clinical trials currently testing such tools is the PROBE trial, which is investigating the first imaging agents designed to be used for direct visualization of α-synuclein (αSyn) and TAR DNA-binding protein 43 (TDP-43) in the retina. These are established protein biomarkers for Parkinson disease (PD) and amyotrophic lateral sclerosis (ALS), respectively.5
In October 2022, Amydis Inc, a privately held company, announced that patient enrollment for the phase 1/2a Prospective Randomized Open, Blinded Endpoint (PROBE) trial was under way. The trial is designed to evaluate a “retinal tracer” — a fluorescent, nonradioactive retinal imaging agent (AMDXP-2011P) that targets protein biomarkers in patients who have PD or ALS. The PROBE study is examining the safety, tolerability, pharmacokinetics, and activity of a single intravenous bolus of AMDXP-2011P in patients with PD and ALS. Designed for use with currently available ocular imaging devices, the imaging agent is capable not only of detecting but also of quantifying deposits of αSyn and TDP-43 (Figure 1).
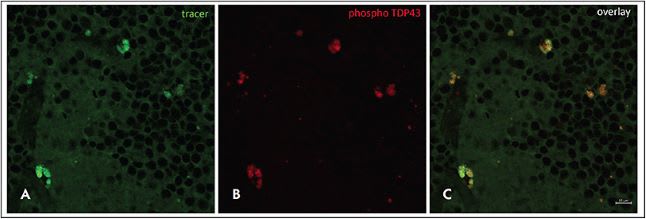
A few weeks after announcing patient enrollment for the PROBE trial, which is being conducted at 2 sites in southern California, Amydis announced the receipt of a third grant, in the amount of $1.5 million, from the Michael J. Fox Foundation for Parkinson’s Research (MJFF). The grant is intended to support the first-in-human study of a retinal tracer targeting αSyn.
“With currently available imaging devices, we have been able to greatly improve our ability to evaluate and manage a host of both retinal and systemic diseases,” says Steven D. Schwartz, MD, Ahmanson Chair, professor of ophthalmology, and chief of the retina division at the UCLA Jules Stein Eye Institute. “By evaluating the retina, we are able to assess vasculature, microvasculature, and neural changes in CNS tissue. Uniquely, the eye allows these evaluations without a biopsy, noninvasively. Although there are currently no approved diagnostics to detect retinal biomarkers in diseases such as PD and ALS, the question of whether or not we will be able to diagnose and manage such neurologic diseases by imaging the retina has been raised, and the answer appears to be a strong yes.”
POTENTIAL USES OF RETINAL TRACERS IN CLINICAL PRACTICE AND RESEARCH
Once approved, retinal tracers will likely be used initially to confirm the diagnoses of individuals with various neurodegenerative diseases.
“A referral to a retina specialist or ophthalmologist who has the appropriate equipment for testing will likely be initiated by a neurologist,” says David S. Boyer, MD, adjunct clinical professor of ophthalmology at the University of Southern California and senior partner at Retina-Vitreous Associates Medical Group in Los Angeles. “Neurologists with patients who have a strong family history of PD, or neurologists with patients suspected of having ALS, for example, could benefit from the information obtained with retinal tracers, which involves a procedure that is much more convenient and much less expensive when compared with PET scans.”
The procedure for using retinal tracers to detect neurologic disease will likely be similar, if not identical, to the process currently used for obtaining images with fluorescein angiography.
“It is possible that eventually specific filters may be required to better delineate areas in which the tracers are showing up,” says Dr. Boyer. “I don’t envision that the tracers will be the same for every disease, so retina specialists and ophthalmologists may need to fine-tune which filters will lead to the best results and allow us to best image specific lesions.”
Dr. Boyer and Dr. Schwartz both anticipate the eventual use of retinal tracers in conjunction with optical coherence tomography (OCT) and other imaging modalities to obtain more detailed information. A combination of imaging technologies could be used to create an array of highly informative molecular, structural, and vascular maps.
“In the future, AI will probably be able to analyze information from the retinal tracers in combination with information obtained from OCTs and arrive at a relative risk factor score — one that takes into consideration variables such as the degree of nerve fiber layer thinning and changes in the ganglion layer. As we move into the future, AI will likely play a bigger role, especially as we obtain more and larger data sets and as AI develops greater ability to identify high-risk or low-risk patients based on analyses of data. A number of researchers — including those at Duke University — are already conducting such analyses,” says Dr. Boyer.
Once retinal tracers are approved, they also should prove to be a significant boon in the realm of drug development.
“In combination with OCT and other imaging modalities, retinal tracers may greatly refine our ability to improve inclusion and exclusion criteria in clinical trials,” says Dr. Schwartz. “They also should significantly facilitate our ability to evaluate the progression of disease and to assess the treatment effects of various drugs or biologics. The potential for more accurately tracking the progress of patients throughout their treatment journey is exciting.”
FUTURE DIRECTIONS
Although in the future retinal tracers might give retinal physicians the ability to screen patients for various neurologic diseases, for now, such use of the tracers is not practical.
“At this time, retinal physicians are unlikely to begin to arbitrarily screen people with these tracers, particularly without any idea as to whether a particular patient is likely to have a neurodegenerative disease,” says Dr. Boyer. “Certainly, Medicare and other insurance companies do not currently pay for such screening.”
Dr. Boyer also notes that further validation of retinal tracers will involve methods for ascertaining the functional benefits of treatments used to address various neurodegenerative diseases. “Although a biomarker may show changes on imaging that may hint that a particular drug is resulting in a benefit, the validation of the biomarker also must involve confirmation that a change in the biomarker correlates with functional improvement,” says Dr. Boyer. A drug for a condition such as Alzheimer disease, for example, could result in the removal of plaques without improving a patient’s ability to function. A drug for Alzheimer disease would have to show some improvement in cognition also. Likewise, a drug for ALS that results in promising changes in a retinal biomarker must also demonstrate a functional improvement, whether that involves lengthening the life of an ALS patient, or giving that patient greater physical functioning. “The validation of a biomarker is always going to come down to function,” he says.
Once retinal tracers are approved, however, the uses for such tracers will likely expand, and eventually they may be used to detect a wide range of proteinopathies and neurodegenerative diseases. Indeed, many in the field of Alzheimer disease research already anticipate that retinal biomarkers will be used to identify preclinical stages of Alzheimer disease and facilitate the use of interventions designed to stop the disease or slow its progression.6 Current biomarker tools used in the Alzheimer disease field, such as amyloid PET imaging and CSF testing, tend to be limited in their availability, expensive, time-consuming, and more invasive than retinal tracers.
“Retinal physicians and ophthalmologists aim to evaluate the whole patient,” says Dr. Schwartz, “and we already are intimately involved with multiple subspecialists and generalists to improve outcomes for our patients, whether they have diabetes or hypertension or collagen vascular diseases or cancer. Some patient conditions are first diagnosed in the eye; in these cases we refer patients out to other specialists. In other cases, conditions are diagnosed by outside specialists and referred in. Regardless of the referral patterns, the availability of exciting new tools such as retinal tracers should be a real step forward when it comes to improving our ability to care for our patients.” RP
Editor’s note: Listen to discussion of this article on the Retina Podcast at www.retinapodcast.com .
REFERENCES
- Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797-812. doi:10.1016/S1474-4422(17)30278-8
- La Morgia C, Ross-Cisneros FN, Sadun AA, Carelli V. Retinal ganglion cells and circadian rhythms in Alzheimer’s disease, Parkinson’s disease, and beyond. Front Neurol. 2017;8:162. doi:10.3389/fneur.2017.00162
- Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018;33(8):1315-1324. doi:10.1002/mds.27392
- Constable PA, Marmolejo-Ramos F, Gauthier M, Lee IO, Skuse DH, Thompson DA. Discrete wavelet transform analysis of the electroretinogram in autism spectrum disorder and attention deficit hyperactivity disorder. Front Neurosci. 2022;16:890461. doi:10.3389/fnins.2022.890461
- AMDX-2011P retinal tracer in subjects with neurodegenerative diseases associated with amyloidogenic proteinopathy (PROBE). ClinicalTrials.gov Identifier: NCT05542576. Updated October 26, 2022. Accessed November 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05542576
- Snyder PJ, Alber J, Alt C, et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement. 2021;17(1):103-111. doi: 10.1002/alz.12179.








