The field of surgical retina has been steadily evolving over the last 80 years, with several paradigm shifts occurring in waves in between periods of calm, rising waters. Arguably, the first major paradigm shift started with scleral buckling in the late 1940s, with the second wave of discovery continuing with open-sky and large-gauge vitrectomy surgery in the 1970s. The third wave occurred in the 1990s with the addition of small-gauge vitrectomy surgery. Currently, the worlds of medical and surgical retina are rapidly expanding as the search for improved efficacy and durability have led to groundbreaking new therapies including small molecules, biologics, cell therapy, and gene therapy. These brilliant medical discoveries have made way for the next wave of vitreoretinal surgery: subretinal cell, gene, and drug delivery.
There has been an effort across the industry to make delivery of these promising agents more precise, more efficient, and safer. The biggest challenges in surgical delivery include reaching the target tissue (cell type) with minimal off-target effects, delivering a specific targeted dose, controlling the speed of delivery, and limiting egress into the vitreous cavity, all of which can lead to reduced therapeutic concentrations, higher rates of intraocular inflammation, cellular dysfunction, teratoma formation, and iatrogenic complications such as macular hole and retinal detachments.1,2 This article will provide an update on subretinal delivery devices that are currently available for the surgical retina specialist for use both with and without a vitrectomy.
TRANSSCLERAL OR TRANSVITREAL CANNULA APPROACH WITH VITRECTOMY
Small-gauge subretinal cannulas for intraocular use during a pars plana vitrectomy are well established, with submacular hemorrhage displacement being the most common indication.3 After a complete vitrectomy, the main entry point is through pars plana cannulas transsclerally, traversing through the vitreous cavity, and then subsequent access by penetration transretinally. Cannulas are available from various manufacturers, ranging in size from 38 gauge to 41 gauge, with polyamide or metal tips (up to 48 gauge). Higher-order versions include extendable or retractable features, such as the 38-gauge Subretinal Polytip, 38-gauge Extendable Polytip Cannula, and 40-gauge Microtip Cannula from MedOne Surgical. These cannulas can be attached to a syringe via extension tubing, so that manual injections can be performed with an assistant.
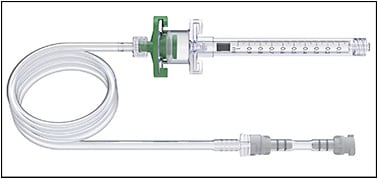
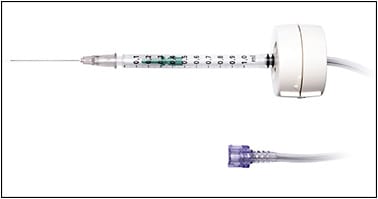
Most recently, these subretinal cannulas have been integrated into the vitrectomy machine for better automated pneumatic control, whereby the cannula is connected to the viscous fluid infusion and viscous fluid control portion of the machine and the surgeon can control the rate of injection using foot pedal control without an assistant. The main commercial devices available are the Microdose Injector by MedOne Surgical (Figure 1) and the Inicio Microinjection system by DORC (Figure 2). Both manual and automated subretinal cannula devices are used in combination with a pars plana vitrectomy.
SUPRACHOROIDAL CANNULA APPROACH WITHOUT VITRECTOMY
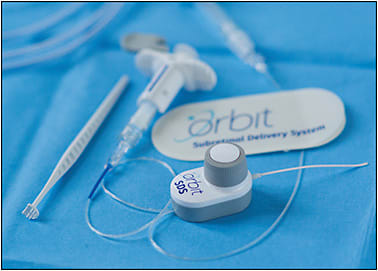
The second mode of subretinal delivery is through a suprachoroidal approach, whereby cannulas traverse the suprachoroidal space with penetration through Bruch’s membrane and the retinal pigment epithelium to access the subretinal space. The Orbit SDS device (formerly Gyroscope Therapeutics, now Novartis) (Figure 3) is the mainstay suprachoroidal device that received FDA 501(k) clearance in August 2020. The Orbit SDS has been successfully used in a variety of gene therapy trials, including Gyroscope Therapeutics’ phase 1/2 FOCUS dry AMD trial. The specific technique has been previously described.4,5 The device contains a 1.6-mm elongated cannula attached to a microdose injector that can be used with viscous fluid control. Initial incisions require a 3-mm sclerotomy to access the suprachoroidal space and the 1.6-mm elongated cannula is advanced through the suprachoroidal space via direct chandelier-assisted visualization. The suprachoroidal device is accompanied by a magnet for better stabilization during injection. Once the cannula is advanced, a microneedle is advanced into the subretinal space through direct visualization and a preparatory subretinal bleb with BSS is injected first before drug or gene delivery. This device may be used independent of a vitrectomy, reducing vitrectomy-related complications and egress into the vitreous cavity from a retinotomy. This device should not be used in patients with certain implanted surgical devices, such as pacemakers.
TRANSSCLERAL TRANSVITREAL CANNULA APPROACH WITH OR WITHOUT VITRECTOMY
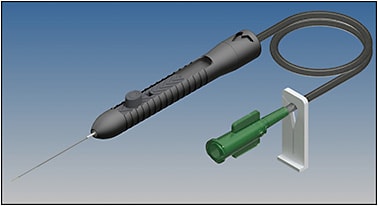
The Nano Subretinal Gateway Device (Vortex Surgical) (Figure 4) is the most recent commercially available subretinal delivery hybrid device. It provides access to the subretinal space through a transcleral approach either with or without a vitrectomy. The surgical technique (Video 1) was described by Wood et al in 2022.6 Current indications include submacular hemorrhage displacement, gene or drug delivery, and, most recently, tumor biopsies. Using a chandelier for direct microscopic visualization, the subretinal device contains a 28-gauge needle attached to a microdose injector that is inserted through a self-sealing transscleral wound via the pars plana. The subretinal device comes in straight or curved versions with an extendable 41-gauge polyamide beveled tip that is further enveloped at the shaft by a stiffened 33-gauge base extendable needle. This specific design provides rigid control during retinal penetration with a goal of reduced vitreous disruption. The manually controlled extendibility feature allows delivery of the therapeutic away from the retinotomy entry site to reduce the risk of egress through the small retinotomy site. The microdose injector can be used with or without an assistant by attaching to the viscous fluid control.
TIPS FOR BEST SURGICAL USE OF THE NANO SUBRETINAL GATEWAY DEVICE:
- Insert chandelier inferiorly to avoid shadowing over the macula.
- Stabilize the eye and enter pars plana similarly to intravitreal injections.
- When close to the retina, extend tip and penetrate.
- If using automated infusion pressure, maintain pressure at 6 to 10 PSI.
- If using the curved version, extend further to access specific subretinal space.
- Inject therapeutic, then when injection is complete, retract polyamide cannula completely, and remove it from the subretinal space and vitreous cavity.
- For further safety, perform scleral depression to ensure no retinal tears are noted in the periphery.
CONCLUSION
In the era of drug, cell, and gene therapy, another exciting wave has hit the surgical retina world — the shift toward surgical subretinal delivery and the pursuit of more precise, efficient, and safe delivery of therapeutic to the retina. Several commercially available subretinal delivery devices are promising with access through a transscleral/transvitreal or suprachoroidal approach with or without vitrectomy. RP
REFERENCES
- Peng Y, Tang L, Zhou Y. Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58(4):217-226. doi:10.1159/000479157
- Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFc1-ORSFc9. doi:10.1167/iovs.15-18681
- Martel JN, Mahmoud TH. Subretinal pneumatic displacement of subretinal hemorrhage. JAMA Ophthalmol. 2013;131(12):1632-1635. doi:10.1001/jamaophthalmol.2013.5464
- Gray AP, Sato Y, Meyer T, Stoner K, Beltran WA. Surgical procedure and applicability of the Orbit Subretinal Delivery System (SDS) in the normal adult canine eye. Invest Ophthalmol Vis Sci. 2022;63:4118–F0355.
- Orbit SDS Delivery System instructions for use. Accessed February 26, 2023. https://www.orbitsds.com/wp-content/uploads/2020/08/AW1009028-Rev.-D-US-IFU-No-Cut-Lines.pdf
- Wood EH, Rao P, Mahmoud TH. Nanovitreoretinal subretinal gateway device to displace submacular hemorrhage: access to the subretinal space without vitrectomy. Retina. 2022;42(11):2225-2228. doi:10.1097/IAE.0000000000002669








