Approximately 5.9 million intravitreal injections (IVI) were performed in the United States in 2016, and estimates predict an annual increase of 10% to continue into the foreseeable future.1 Postinjection endophthalmitis (PIE) is the most significant and potentially sight-threatening complication of IVI, with an incidence ranging from 0.028% to 0.056% (1 in 1,780 to 3,544 injections) in 4 meta-analyses (Figure 1).2-5 However, a recent study reported a lower incidence of 10 cases of PIE in 71,858 IVI (1 PIE in 7,186 injections, 0.014%).6 Although a recent meta-analysis failed to detect a significant difference in the overall incidence of PIE after IVI performed in the office or operating room (OR) settings, PIE after IVI in an office setting was more likely to be culture positive.7 Significantly lower incidences of PIE were observed in some studies where IVI were administered in the OR: 0 PIE in 20,293 IVI,8 65 PIE in 316,576 IVI or 1 PIE per 4,762 IVI (0.021%),9 and 10 PIE in 134,701 IVI (0.007%).10
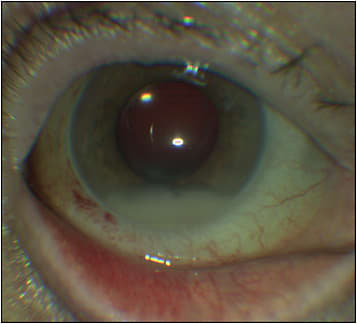
Considering the preponderance of coagulase-negative Staphylococci (CNS), a normal commensal of the ocular surface, as a causative organism in PIE, superior air quality in the OR may not be the likely explanation for observed superiority of OR over office. It is plausible that patients receiving IVI in an OR receive more attention to antisepsis (better prepping of ocular surface, lid margins, eye lashes, and periocular regions and better technique such as universal speculum use) compared to those receiving them in an office.11 Other risk factors for PIE include triamcinolone (4-10 times increased risk compared to anti-VEGF injections),12,13 dexamethasone implant (Ozurdex from Allergan/AbbVie; 0.102% or 1 in 981 injections),14 blepharitis,15 and omission of povidone-iodine (PI).16,17 Prefilled syringes may reduce the risk of PIE.18 A large retrospective study of 4.3 million IVIs in the Vestrum Health database from January 2013 through June 2019 revealed 0.049% incidence of PIE for intravitreal aflibercept, 0.026% for bevacizumab, 0.026% for ranibizumab vial, 0.022% for prefilled ranibizumab, 0.107% for dexamethasone implant, and 0.147% for triamcinolone.19
Bhavsar et al and Rebaldi et al observed 2.5 times and 3 times higher incidence of PIE, respectively, in patients who received topical antibiotics compared to those who did not.2,20 Therefore, topical antibiotics do not protect against PIE and may even be harmful. For best outcomes, IVI should be avoided in patients with active ocular or periocular infections.
BACTERIOLOGY
Oral and nasopharyngeal cavities of personnel are a potential source of pathogens, and there is some evidence that a no-talking policy or face mask use by surgeons may reduce the occurrence of PIE caused by oral flora.21,22 Approximately 40% to 50% of cases of PIE are culture positive,2-4,23 most of them being caused by gram-positive cocci. The most common bacterial isolate in PIE is CNS, followed by oral flora, which have been isolated in 29% to 56% of all culture-positive cases and are associated with worse visual and anatomic outcomes.3,4,23,24 In the Endophthalmitis Vitrectomy Study, incidence of endophthalmitis caused by oral flora after cataract surgery was 9%, which is 3 to 5 times lower than that seen in PIE.
NORMAL CONJUNCTIVAL FLORA
Ratumnoi et al evaluated bacterial isolates from the conjunctival sac and lid margins in 120 patients undergoing cataract surgery and found that CNS were the most common bacteria on the lid margins (58.3%) and conjunctiva (30%), followed by Streptococcus. The rate of bacterial colonization increased with age, hypertension, and diabetes.25 Similarly, Suto et al found higher rates of positive conjunctival cultures in patients older than 60 years. Methicillin-resistant CNS were isolated at a higher rate in patients with diabetes compared to those without diabetes (20% vs 7%).26 Lid margins, eye lashes, and eyelids should be prepped prior to IVI because there are more bacteria in the lid margin than in the conjunctival sac. For the same reason, pressure on the eyelids should be avoided, because it may release bacteria from the lid margins into the conjunctival sac. If globe softening is needed, pressure should be applied directly over the globe and not on the eyelids.27
POVIDONE-IODINE
Povidone-iodine (Betadine; Avrio Health) is a chemical complex of a water-soluble polymer povidone and triiodide. The antimicrobial action of iodine occurs after it dissociates from the complex. Free iodine rapidly penetrates microbial cell membranes and interacts with proteins, nucleotides, and fatty acids in the cytoplasm and cytoplasmic membranes, resulting in rapid cell death within 15 seconds.28 Povidone-iodine is bactericidal, fungicidal, and virucidal, possessing antimicrobial activity against gram-positive and gram-negative bacteria, fungi, protozoa, viruses, and bacterial (but not fungal) spores. Resistance to PI has not been reported. Although it was first synthesized in 1955, it was FDA approved in December 1986 for topical skin disinfection. It is commonly used as surgical hand scrub, topical antiseptic solutions, topical antiseptic cream, sore throat gargle (0.5%), skin prep prior to surgery, and for prepping the ocular surface, lid margins, and periorbital skin prior to ophthalmic surgery.
Current US guidelines recommend administration of 5% to 10% PI at least 30 seconds prior to intravitreal injections.27 It can be safely used in most patients with self-reported iodine or shellfish allergy.29 This is because the so-called allergy to iodinated contrast agents is a reaction to hyperosmolar solutions, and allergy to shellfish is due to tropomyosin, a muscle protein, and not to iodine, a vital micronutrient.28
In 1991, Speaker and Menikoff showed a fourfold decrease in endophthalmitis after cataract surgery with 5% PI vs routine use of a silver nitrate solution.30 Ciulla et al strongly supported the use of PI for preoperative antisepsis in 2002.31 As a result of these and other studies, perioperative use of 5% to 10% PI has become standard of care for all ophthalmic surgical procedures, including IVI.
Topical antibiotics are no longer recommended either before or after IVI.27,32 Patients who received anti-VEGF injections without PI due to self-reported iodine allergy had a very high (5 cases of PIE in 53 injections, 9.4%) endophthalmitis rate in 1 study.17 Other studies have reported significantly higher PIE rates when PI antisepsis was omitted or reduced. A retrospective study by Bhavsar et al showed 15% risk of endophthalmitis per injection.16 Standard protocol (n=21,185 IVI) in Mulcahy et al consisted of 2 drops of 5% PI instilled 3 minutes before injection, followed by cleansing of the periorbital skin with 10% PI and a further drop of 5% PI 1 to 2 minutes before IVI. No PI was administered after IVI. The reduced PI protocol (n=769 IVI) consisted of 1 drop of 5% PI 3 minutes before IVI, followed by cleansing of the skin with cutaneous 0.05% chlorhexidine gluconate. After IVI, the conjunctival sac was washed out with 0.9% normal saline. The patients in the group that received no PI (n=92 IVI) received periorbital skin prep with 0.05% chlorhexidine gluconate only. All groups received a drop of 0.5% chloramphenicol after IVI. The overall incidence of PIE was 0.05% (1 in 2,000 injections). However, the incidence of PIE was 0.78% (39 times higher than 0.02% PIE incidence in standard PI) in the reduced PI group, and 1.09% (54 times compared to standard PI) in the group that received no PI.33
TECHNIQUE
Although the value of PI antisepsis in IVI is well established, there is lack of clarity on the technique (single drop vs multiple drops vs irrigation), concentration of PI, and contact time. A 10 mL flush of the conjunctival sac and fornices with 5% PI provided a greater reduction of bacteria than a 2 to 3 drop application of 5% PI. Microbiological cultures were obtained 2 minutes after PI application prior to IVI.34 Because irrigation of the conjunctival sac with a 10 mL solution will take at least 1 minute, observed benefit of flush over drops may have been due to longer contact time with the former.34
The maximum volume of the conjunctival sac is only 30 μL, about half of the usual 50 μL eye drop.35 Because aqueous tear turnover rate is 10% per minute,36 and a topically applied drop may not reach all parts of the conjunctival sac, it may be reasonable to consider multiple applications of PI. Stranz et al showed that 2 applications of 5% PI separated by 10 minutes were more effective than a single application.37
CONCENTRATION OF POVIDONE-IODINE
Yanai et al studied cytotoxicity of PI on cultured human corneal epithelial cells and observed increase in toxicity with PI concentration and exposure time.38 In rabbit eyes, damage to conjunctival goblet cells and reduction in mucin were noted after contact with 5% PI exceeding 3 minutes.39 Well-documented side effects of topical 5% PI include conjunctival irritation and superficial punctate keratopathy. Intolerance to 5% PI was reported in 2.4% of patients by Mulcahy et al and may be more common in patients with dry eye syndrome.33 A recent study showed improved comfort and reduced ocular surface irritation with dissolvable collagen punctal plugs inserted after IVI with 5% PI prep in patients with moderate to severe symptoms and objective findings of dry eyes.40 Other methods to reduce pain and discomfort from 5% PI solution include topical NSAIDS,41 saline rinse after IVI, and ocular irrigation with 0.01% hypochlorous acid after IVI.42 Vigorous irrigation of ocular surface with normal saline or balanced salt solution may dislodge bacteria from conjunctival fornices and should be avoided.
The bactericidal activity of PI results from free iodine, which is released from the povidone polymer-iodine complex as free iodine is depleted, until all available iodine is exhausted. As PI solution is diluted, the concentration of free iodine paradoxically increases, resulting in faster killing of bacteria up to the dilution of 1:100 (0.1%) compared to the stock solution of 10% PI.43 A recent publication also showed faster killing of a variety of bacterial isolates including methicillin-resistant Staphylococcus epidermidis by 0.6% PI compared to 5% PI.44 Free iodine concentrations in PI solutions are 5 parts per million (ppm) in 10%; 13 ppm in 1%; 24 ppm in 0.1%, and 13 ppm in 0.01%.45 Concentrations less than 0.05% lose their povidone-iodine complex characteristics and behave like an aqueous solution of iodine. Compared to lower concentrations of PI, higher concentrations of PI have more bound iodine reservoir, which will be released as free iodine is used up. Therefore, higher concentrations of PI will be more effective if the bacterial load is high. Ferguson et al irrigated the conjunctival sac with 2 mL solution of either 1% or 5% PI over 1 minute prior to cataract surgery. Conjunctival swabs taken 1 minute after irrigation showed greater reduction in bacterial colony forming units with 5% PI compared to 1%, particularly in eyes with higher initial bacterial load.46 Repeated applications of dilute PI can replenish the iodine reservoir while reducing ocular surface toxicity that is common with higher concentrations. Silas et al showed that 3 applications of 1% PI were as effective as a single application of 5% or 10% in inhibiting the growth of S. epidermidis on blood agar plates.47
There are several reports of successful applications of dilute PI in ophthalmology. Shimada et al evaluated multiple applications of 0.25% PI solution (1 drop prior to draping, followed by ocular irrigation with 5 mL solution after draping and insertion of eyelid speculum, IVI after waiting for 30 seconds, followed by another conjunctival wash with 5 mL solution) and observed 0 cases of PIE after 15,144 injections.48 Khan et al showed equivalent efficacy of 1.25% and 2.5% PI solution in the prevention of ophthalmia neonatorum.49 Isenberg et al demonstrated successful treatment of bacterial keratitis with 1.25% PI solution.50 Repeated intraoperative irrigation of the ocular surface with 0.25% PI every 20 to 30 seconds reduced anterior-chamber and vitreous contamination to 0% after cataract surgery or pars plana vitrectomy, compared to 5% and 2% respectively for eyes treated with single application of PI prior to surgery.51,52 Intravitreal injection of 0.1 mL 1.25% PI solution followed by vitrectomy using 0.025% PI in vitrectomy fluid has been used successfully for the treatment of endophthalmitis.53 Both 1% and 5% PI solutions were superior to gentamicin solution in reducing bacterial load from scleral buckling elements.54 Peden et al showed equivalent efficacy of 0.62%, 1.25%, 2.5%, and 5% PI solutions in preventing PIE.55
Thus, repeated applications of reduced concentrations of PI may be effective in preventing PIE while improving patient comfort. A significant component of ocular irritation from 5% PI is from its acidic pH of 4. Diluting it with a buffered normal saline solution will bring its pH closer to the normal pH of tear film. Thakur et al demonstrated that PI diluted to 1% and adjusted to the pH of 7 retained its antibacterial activity and was stable for up to a month.56
Povidone-iodine solution should be applied prior to giving subconjunctival anesthetic or application of jelly for anesthesia. It should also be the last drop to enter the eye prior to IVI.27 Because bacteria can also enter the eye soon after the IVI, particularly in eyes with thin sclera or scarred conjunctiva, or when a larger bore needle is used, for example for triamcinolone acetonide or steroid implants (dexamethasone or fluocinolone), it may be reasonable to instill 1 or 2 drops of PI after the injection.
CONTACT TIME
The American Academy of Ophthalmology, the American Society of Cataract and Refractive Surgery, and the European Society of Cataract and Refractive Surgery recommend that 5% to 10% solution of PI should be applied to the conjunctival sac, cornea, lid margins, and periorbital skin for at least 3 minutes prior to surgery.57 Current US guidelines recommend a minimum exposure time of 30 seconds prior to IVI,27 but a 2-minute exposure is the recommended protocol in France,9 and 3 minutes is the recommendation in the United Kingdom.58
Although PI is bactericidal in 10 to 15 seconds for most isolates, some isolates may take 10 to 15 minutes.59 Therefore, it may be reasonable to give at least 3 minutes contact time with PI prior to IVI.
GLOVES, DRAPING, AND LID SPECULUM
There is no evidence to support the use of gloves and drapes in the prevention of PIE. However, gloves, sterile or nonsterile, may be worn according to personal preference.27
A solid blade speculum is the most effective method to prevent contact of the eyelashes and lid margin with the injection site and the injection needle. Some surgeons use manual lid retraction by an assistant to maintain this separation. Having the patient look in the opposite direction from the quadrant of IVI will also reduce the risk of the needle touching the lid margin or eyelashes (Figure 2).

AFTERCARE
Patients should be advised not to touch their eyes with dirty hands or apply a tissue to the eye. They should also be instructed to avoid eye rubbing on the day of injection.
ALTERNATIVES TO POVIDONE-IODINE
Aqueous chlorhexidine gluconate (AqCXH) 0.05% to 0.1% is an acceptable alternative to PI and may cause less pain and discomfort compared to 5% PI.60-62 However, it is not readily available; can cause corneal toxicity and allergic reactions such as contact dermatitis, urticaria, dyspnea and anaphylactic shock; and carries a warning from the manufacturer: “Do not irrigate brain, meninges, eyes or perforated eardrum.”61 Moreover, it is a clear solution and lacks the visual feedback of PI solution.
Hypochlorous acid (0.01%) has been considered as a substitute for PI but needs further study. A recent publication found it to be as effective as 5% PI in reducing bacterial colony forming units (CFUs) after 1 minute exposure without any ocular discomfort in a small study involving 40 patients.63 However, 1 minute contact time with PI might not be adequate, and eyes in the PI group were “rinsed with sterile saline” which could have increased bacterial CFUs in the PI group by dislodging bacteria. Another publication found it to ineffective in ocular surface antisepsis.64 At present, there is not enough evidence to recommend alternatives to PI or AqCXH for ocular surface antisepsis.65
CONCLUSION
An abundance of literature supports PI antisepsis for IVI. When performing IVI, all personnel should wear face masks and minimize talking. The patient should also be advised to minimize talking during the procedure. Although not proven, redirected air flow toward the eye from a poorly sealed mask worn by the patient could increase the risk of contamination of the injection site by the patient’s breath. Retina specialists can consider using dilute PI particularly in patients with dry eyes or history of significant pain and discomfort following previous IVI. In the authors’ experience, PI solution in a concentration of 1.7% or less causes minimal irritation and can be applied to the conjunctiva without prior anesthetic agent; we switched to 1.7% PI more than 2 years ago without any PIE in more than 10,000 intravitreal injections.66 The consensus across anterior-segment literature is to give at least 3 minutes of contact time with PI prior to intravitreal injection. Evidence also supports antiseptic benefits of multiple drops of PI before and immediately after IVI over a span of several minutes. Consider using a solid blade speculum to prevent the lashes and lid margins from contacting the needle or the injection site and have the patient look in the opposite direction of the quadrant of intravitreal injection (Figure 2). Consider taking additional precautions with dexamethasone implant and intravitreal triamcinolone. These measures may include conjunctival displacement, oblique entry, pressure on the injection site with a sterile cotton-tipped applicator or a second instrument for 10 seconds to help seal the wound, PI drops a couple of times after IVI, and patching for a few hours. RP
REFERENCES
- Williams GA. IVT injections: health policy implications. Review of Ophthalmology. June 5, 2014. Accessed February 24, 2023. https://www.reviewofophthalmology.com/article/ivt-injections-health-policy-implications
- Reibaldi M, Pulvirenti A, Avitabile T, et al. Pooled estimates of incidence of endophthalmitis after intravitreal injection of anti–vascular endothelial growth factor agents with and without topical antibiotic prophylaxis. Retina. 2018;38(1):1-11. doi:10.1097/IAE.0000000000001583
- McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti–vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654-661. doi:10.1097/IAE.0b013e31820a67e4
- Fileta JB, Scott IU, Flynn HW. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti–vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014;45(2):143-149. doi:10.3928/23258160-20140306-08
- Merani R, Hunyor AP. Endophthalmitis following intravitreal anti–vascular endothelial growth factor (VEGF) injection: a comprehensive review. Int J Retina Vitreous. 2015;1:9. doi:10.1186/s40942-015-0010-y
- Reyes-Capo DP, Yannuzzi NA, Smiddy WE, Flynn HW. Trends in endophthalmitis associated with intravitreal injection of anti-VEGF agents at a tertiary referral center. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):319-326. doi:10.3928/23258160-20210528-04
- Li T, Sun J, Min J, et al. Safety of receiving anti–vascular endothelial growth factor intravitreal injection in office-based vs operating room settings: a meta-analysis. JAMA Ophthalmol. 2021;139(10):1080-1088. doi:10.1001/jamaophthalmol.2021.3096
- Brynskov T, Kemp H, Sørensen TL. No cases of endophthalmitis after 20,293 intravitreal injections in an operating room setting. Retina. 2014;34(5):951-957. doi:10.1097/IAE.0000000000000071
- Dossarps D, Bron AM, Koehrer P, Aho-Glélé LS, Creuzot-Garcher C. Endophthalmitis after intravitreal injections: Incidence, presentation, management, and visual outcome. Am J Ophthalmol. 2015;160(1):17-25.e1. doi:10.1016/j.ajo.2015.04.013
- Freiberg FJ, Brynskov T, Munk MR, et al. Low endophthalmitis rates after intravitreal anti–vascular endothelial growth factor injections in an operating room: a retrospective multicenter study. Retina. 2017;37(12):2341-2346. doi:10.1097/IAE.0000000000001488
- Chaturvedi R, Wannamaker KW, Riviere PJ, Khanani AM, Wykoff CC, Chao DL. Real-world trends in intravitreal injection practices among American retina specialists. Ophthalmol Retina. 2019;3(8):656-662. doi:10.1016/j.oret.2019.03.023
- Baudin F, Benzenine E, Mariet A, et al. Topical antibiotic prophylaxis and intravitreal injections: impact on the incidence of acute endophthalmitis — a nationwide study in France from 2009 to 2018. Pharmaceutics. 2022;14(10):2133. doi:10.3390/pharmaceutics14102133
- Cheung CSY, Wong AWT, Lui A, Kertes PJ, Devenyi RG, Lam W. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119(8):1609-1614. doi:10.1016/j.ophtha.2012.02.014
- Samuelson AG, Nahar A, Patel SN, et al. Clinical outcomes of patients with endophthalmitis after dexamethasone intravitreal implant. Retina. 2022;42(10):1915-1920. doi:10.1097/IAE.0000000000003546
- Lyall DaM, Tey A, Foot B, et al. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond). 2012;26(12):1517-1526. doi:10.1038/eye.2012.199
- Bhavsar AR, Glassman AR, Stockdale CR, Jampol LM. Elimination of topical antibiotics for intravitreous injections and the importance of using povidone-iodine: update from the Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol. 2016;134(10):1181-1183. doi:10.1001/jamaophthalmol.2016.2741
- Modjtahedi BS, van Zyl T, Pandya HK, Leonard RE, Eliott D. Endophthalmitis after intravitreal injections in patients with self-reported iodine allergy. Am J Ophthalmol. 2016;170:68-74. doi:10.1016/j.ajo.2016.07.010
- Storey PP, Tauqeer Z, Yonekawa Y, et al. The impact of prefilled syringes on endophthalmitis following intravitreal injection of ranibizumab. Am J Ophthalmol. 2019;199:200-208. doi:10.1016/j.ajo.2018.11.023
- Dhoot DS, Boucher N, Pitcher JD, Saroj N. Rates of suspected endophthalmitis following intravitreal injections in clinical practices in the United States. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):312-318. doi:10.3928/23258160-20210528-03
- Bhavsar AR, Googe JM, Stockdale CR, et al. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: the Diabetic Retinopathy Clinical Research Network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol. 2009;127(12):1581-1583. doi:10.1001/archophthalmol.2009.304
- Patel SN, Hsu J, Sivalingam MD, et al. The impact of physician face mask use on endophthalmitis after intravitreal anti–vascular endothelial growth factor injections. Am J Ophthalmol. 2021;222:194-201. doi:10.1016/j.ajo.2020.08.013
- Ong AP, Angbue Te N, Zagora SL, et al. Post-surgical versus post-intravitreal injection endophthalmitis: changing patterns in causative flora. Clin Exp Ophthalmol. 2019;47(1):57-62. doi:10.1111/ceo.13345
- Garg SJ, Dollin M, Storey P, et al. Microbial spectrum and outcomes of endophthalmitis after intravitreal injection vs pars plana vitrectomy. Retina. 2016;36(2):351-359. doi:10.1097/IAE.0000000000000694
- Simonett JM, Igelman A, Taylor SC, et al. Culture-proven endophthalmitis after intravitreal injection: a 10-year analysis. Ophthalmic Surg Lasers Imaging Retina. 2019;50(1):33-38. doi:10.3928/23258160-20181212-05
- Ratnumnoi R, Keorochana N, Sontisombat C. Normal flora of conjunctiva and lid margin, as well as its antibiotic sensitivity, in patients undergoing cataract surgery at Phramongkutklao hospital. Clin Ophthalmol. 2017;11:237-241. doi:10.2147/OPTH.S109247
- Suto C, Morinaga M, Yagi T, Tsuji C, Toshida H. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect Drug Resist. 2012;5:37-41. doi:10.2147/IDR.S27937
- Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34 Suppl 12:S1-S18. doi:10.1097/IAE.0000000000000399
- Stewart MW. Doctor I have an iodine allergy. Ophthalmol Ther. 2022;11(3):931-938. doi:10.1007/s40123-022-00502-1
- Wykoff CC, Flynn HW, Han DP. Allergy to povidone-iodine and cephalosporins: the clinical dilemma in ophthalmic use. Am J Ophthalmol. 2011;151(1):4-6. doi:10.1016/j.ajo.2010.08.044
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98(12):1769-1775. doi:10.1016/s0161-6420(91)32052-9
- Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109(1):13-24. doi:10.1016/s0161-6420(01)00899-5
- Torres-Costa S, Ramos D, Brandão E, et al. Incidence of endophthalmitis after intravitreal injection with and without topical antibiotic prophylaxis. Eur J Ophthalmol. 2021;31(2):600-606. doi:10.1177/1120672120902028
- Mulcahy LT, Schimansky S, Fletcher E, Mohamed Q. Post-injection endophthalmitis rates with reduced povidone-iodine prophylaxis in patients with self-reported iodine sensitivity. Eye (Lond). 2021;35(6):1651-1658. doi:10.1038/s41433-020-01145-x
- Safar A, Dellimore MC. The effect of povidone iodine flush versus drops on conjunctival colonization before intravitreal injections. Int Ophthalmol. 2007;27(5):307-312. doi:10.1007/s10792-007-9073-6
- Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol. 1966;5(3):264-276.
- Mochizuki H, Yamada M, Hatou S, Tsubota K. Turnover rate of tear-film lipid layer determined by fluorophotometry. Br J Ophthalmol. 2009;93(11):1535-1538. doi:10.1136/bjo.2008.156828
- Stranz CV, Fraenkel GE, Butcher AR, Esterman AJ, Goggin MJ. Survival of bacteria on the ocular surface following double application of povidone-iodine before cataract surgery. Eye (Lond). 2011;25(11):1423-1428. doi:10.1038/eye.2011.182
- Yanai R, Yamada N, Ueda K, et al. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: antimicrobial activity and cytotoxicity for corneal epithelial cells. Cont Lens Anterior Eye. 2006;29(2):85-91. doi:10.1016/j.clae.2006.02.006
- Kim S, Ahn Y, Lee Y, Kim H. Toxicity of povidone-iodine to the ocular surface of rabbits. BMC Ophthalmol. 2020;20(1):359. doi:10.1186/s12886-020-01615-6
- Jin HD, Surbeck JW, Marsh HR, et al. The effect of punctal plugs in reducing ocular surface irritation after povidone-iodine preparation of intravitreal injection — a randomized trial. Eye (Lond). 2022;36(3):568-574. doi:10.1038/s41433-021-01476-3
- Popovic MM, Muni RH, Nichani P, Kertes PJ. Topical nonsteroidal anti-inflammatory drugs for pain resulting from intravitreal injections: a meta-analysis. Ophthalmol Retina. 2020;4(5):461-470. doi:10.1016/j.oret.2020.01.024
- Fam A, Finger PT, Tomar AS, Garg G, Chin KJ. Hypochlorous acid antiseptic washout improves patient comfort after intravitreal injection: a patient-reported outcomes study. Indian J Ophthalmol. 2020;68(11):2439-2444. doi:10.4103/ijo.IJO_2001_20
- Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982;15(4):635-639. doi:10.1128/jcm.15.4.635-639.1982
- Musumeci R, Bandello F, Martinelli M, Calaresu E, Cocuzza CE. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur J Ophthalmol. 2019;29(6):673-677. doi:10.1177/1120672118802541
- Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986;151(3):400-406. doi:10.1016/0002-9610(86)90477-0
- Ferguson AW, Scott JA, McGavigan J, et al. Comparison of 5% povidone-iodine solution against 1% povidone-iodine solution in preoperative cataract surgery antisepsis: a prospective randomised double blind study. Br J Ophthalmol. 2003;87(2):163-167. doi:10.1136/bjo.87.2.163
- Silas MR, Schroeder RM, Thomson RB, Myers WG. Optimizing the antisepsis protocol: effectiveness of 3 povidone-iodine 1.0% applications versus a single application of povidone-iodine 5.0%. J Cataract Refract Surg. 2017;43(3):400-404. doi:10.1016/j.jcrs.2017.01.007
- Shimada H, Hattori T, Mori R, Nakashizuka H, Fujita K, Yuzawa M. Minimizing the endophthalmitis rate following intravitreal injections using 0.25% povidone-iodine irrigation and surgical mask. Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1885-1890. doi:10.1007/s00417-013-2274-y
- Khan FA, Hussain MA, Khan Niazi SP, Haq Zu, Akhtar N. Efficacy of 2.5% and 1.25% povidone-iodine solution for prophylaxis of ophthalmia neonatorum. J Coll Physicians Surg Pak. 2016;26(2):121-124.
- Isenberg SJ, Apt L, Valenton M, et al. Prospective, randomized clinical trial of povidone-iodine 1.25% solution versus topical antibiotics for treatment of bacterial keratitis. Am J Ophthalmol. 2017;176:244-253. doi:10.1016/j.ajo.2016.10.004
- Shimada H, Arai S, Nakashizuka H, Hattori T, Yuzawa M. Reduction of anterior chamber contamination rate after cataract surgery by intraoperative surface irrigation with 0.25% povidone-iodine. Am J Ophthalmol. 2011;151(1):11-17.e1. doi:10.1016/j.ajo.2010.07.002
- Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Reduction of vitreous contamination rate after 25-gauge vitrectomy by surface irrigation with 0.25% povidone-iodine. Retina. 2013;33(1):143-151. doi:10.1097/IAE.0b013e318261a6ce
- Nakashizuka H, Shimada H, Hattori T, Tanaka K, Kitagawa Y, Shinoda K. Intravitreal injection of 1.25% povidone iodine followed by vitrectomy using 0.025% povidone iodine irrigation for treating endophthalmitis. Transl Vis Sci Technol. 2019;8(1):21. doi:10.1167/tvst.8.1.21
- Lin X, Le B, Lee P, Abrams GW, Juzych M, Kumar A. Comparison of povidone-iodine and gentamicin soak as scleral buckle infection prophylaxis. Clin Ophthalmol. 2021;15:2203-2209. doi:10.2147/OPTH.S305637
- Peden MC, Hammer ME, Suñer IJ. Dilute povidone-iodine prophylaxis maintains safety while improving patient comfort after intravitreal injections. Retina. 2019;39(11):2219-2224. doi:10.1097/IAE.0000000000002290
- Thakur SS, Bai A, Chan D, et al. Ex vivo evaluation of the influence of pH on the ophthalmic safety, antibacterial efficacy, and storage stability of povidone-iodine. Clin Exp Optom. 2021;104(2):162-166. doi:10.1111/cxo.13100
- Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data dilemmas and conclusions. European Society of Cataract and Refractive Surgeons. Updated 2018. . Accessed Feb 13, 2023. https://www.escrs.org/media/uljgvpn1/english_2018_updated.pdf
- Intravitreal injection therapy. The Royal College of Ophthalmologists. Updated August 2018. Accessed March 17, 2023. https://www.rcophth.ac.uk/wp-content/uploads/2022/02/Intravitreal-Injection-Therapy-August-2018-1.pdf
- Hosseini H, Ashraf MJ, Saleh M, et al. Effect of povidone-iodine concentration and exposure time on bacteria isolated from endophthalmitis cases. J Cataract Refract Surg. 2012;38(1):92-96. doi:10.1016/j.jcrs.2011.06.030
- Ali FS, Jenkins TL, Boparai RS, et al. Aqueous chlorhexidine compared with povidone-iodine as ocular antisepsis before intravitreal injection: a randomized clinical trial. Ophthalmol Retina. 2021;5(8):788-796. doi:10.1016/j.oret.2020.11.008
- Merani R, McPherson ZE, Luckie AP, et al. Aqueous chlorhexidine for intravitreal injection antisepsis: a case series and review of the literature. Ophthalmology. 2016;123(12):2588-2594. doi:10.1016/j.ophtha.2016.08.022
- Oakley CL, Vote BJ. Aqueous chlorhexidine (0.1%) is an effective alternative to povidone-iodine for intravitreal injection prophylaxis. Acta Ophthalmologica. 2016;94(8):e808-e809. doi:10.1111/aos.12981
- Hejkal TW, Maloley LA, Kaddoura L. Hypochlorous acid 0.01% vs povidone-iodine 5% for ocular antisepsis. J Vitreoret Dis. 2022;6(2):132-137. doi:10.1177/24741264211013622
- Grzybowski A, Kanclerz P. Letter to the editor: evolving guidelines for intracameral injection. J Glaucoma. 2021;30(3):e123. doi:10.1097/IJG.0000000000001708
- Kanclerz P, Myers WG. Potential substitutes for povidone-iodine in ocular surgery. Eye (Lond). 2021;35(10):2657-2659. doi:10.1038/s41433-021-01447-8
- Kishore K. Noninferiority of 1.7% povidone-iodine for endophthalmitis prophylaxis after intravitreal injections compared with 5% povidone-iodine in an office setting. Presented at: the American Society of Retina Specialists 40th annual meeting; July 16, 2022; New York, NY.








