Choroidal melanoma is the most common primary intraocular tumor. Based on the Collaborative Ocular Melanoma Study (COMS) classification, one of two size-based classification systems, it is classified into 3 sizes: small (1-2.5 mm in thickness, 5-16 mm in the largest basal dimension), medium (2.5-10 mm in thickness, 16 mm in the largest basal dimension) and large (>10 mm in thickness, >16 mm in the largest basal dimension).1,2 Choroidal nevus was described as <1 mm in thickness and <5 mm in size.1,2
Considering how choroidal nevus evolves into melanoma, there is an overlap in size between nevus and melanoma without absolute cutoffs.3 Over the years, several studies have explored clinical and multimodal imaging features to differentiate the choroidal nevus from the small choroidal melanoma.4-6 Tumor thickness >2 mm, subretinal fluid, orange pigment, tumor location <3 mm to the optic disc, presence of visual symptoms, and ultrasonographic hollowness are well established risk factors for growth.4-6 These measures are used as surrogate for predicting the growth and hence the diagnosis of choroidal melanoma, whereas the presence of drusen or intraretinal fluid shows chronicity of the lesion that favors the diagnosis of choroidal nevus.4-6 The term “indeterminate lesion” is used for borderline lesions that cannot be definitively diagnosed as melanoma but require close follow-up to confirm that the lesion is already a small melanoma or has evolved into melanoma.7
Plaque radiotherapy is currently the most used treatment for small choroidal melanomas and indeterminate lesions. Because radiation lacks tumor tissue specificity, it may cause irreversible retinal damage and vision loss.8,9 Some eyes may require enucleation due to tumor recurrence or radiation-related side effects. A review of 1,780 small choroidal melanomas (3 mm in thickness) treated with plaque radiotherapy showed a local tumor control rate of 93.5% at 5 years and 90% at 10 years.10
By Kaplan-Meier analysis, visual loss following plaque radiotherapy (3 Snellen lines) was 10% at 1 year, 39% at 5 years, and 49% at 10 years; severe vision loss (20/200 or >6 Snellen lines) was 7% at 1 year, 39% at 5 years, and 54% at 10 years.10 The need for enucleation was 4% at 5 years and 7.6% at 10 years.10 Melanoma-associated metastasis was observed 0.2% at 1 year, 4.5% at 5 years, and 8.8% at 10 years.10 There is a high unmet need for a new vision-preserving first-line treatment option for indeterminate lesions and small choroidal melanomas.
VIRUSES AS ANTICANCER AGENTS
AU-011 (belzupacap sarotalocan) is a novel virus-like drug conjugate (VDC), the first-in-class investigational targeted therapy in clinical development for the primary treatment of indeterminate lesions and small choroidal melanomas. AU-011 can be delivered via intravitreal or suprachoroidal injections. The phase 2 clinical trial via suprachoroidal injection is currently recruiting patients;11 the phase 1b/2 clinical trial via intravitreal injection has completed the patient recruitment and 12-month follow-up.12
Virus-like particles (VLPs) are bionanomaterials that use the biocompatibility of viruses for the development of therapeutics, vaccines, and imaging tools.13,14 They are composed of recombinant capsid proteins that may be engineered to deliver drugs efficiently to tumors. They are not associated with an increased biosafety risk, because they lack viral genetic material and have no proliferative potential.14 VLPs derived from the human papillomavirus were reported to have a strong, restricted tropism to bind and infect most tumor-derived ovarian, lung, and melanoma cell lines in vitro and have analogous tumor-specific properties in vivo after local or intravenous injection.15 The tumor specificity of these VLPs is driven by its binding to modified heparan sulfate proteoglycans (HSPGs) found on the tumor cell surface.15 Tumor cells evolve HSPG modification patterns that mimic the pattern normally found on the basement membrane of damaged epithelial tissues. It has been described that N-sulfation and, to a lesser degree, 6-O sulfation, are important sulfation patterns for the binding specificity of the VLP to this unique cancer cell receptor.15-17
MECHANISM OF ACTION OF NOVEL INFRARED DYE-CONJUGATED VIRUS-LIKE DRUG CONJUGATE
AU-011 is a VDC based on recombinant VLP derived from the human papillomavirus conjugated to a phthalocyanine-based photosensitizer (Irdye 700DX; LI-COR Biosciences) that is activated by 689-nm near-infrared light (Figure 1).
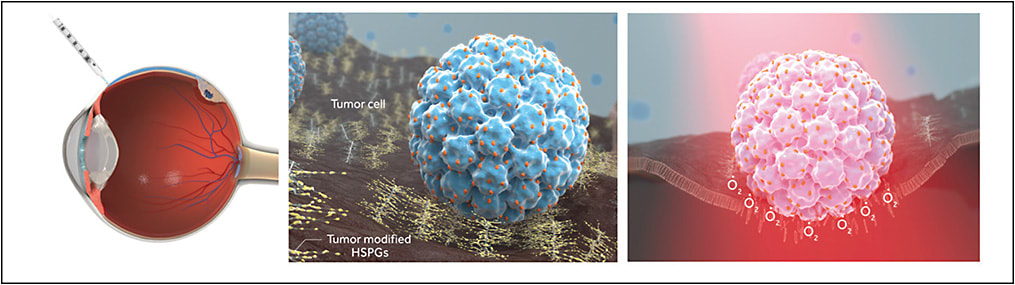
The anti-cancer properties of AU-011 were assessed by using a panel of ovarian, lung, breast, cervical, head and neck, and bladder cancers, and cutaneous and uveal melanoma cell lines in vitro, and AU-011 showed potent and selective anticancer activity.18 Its anticancer activity was blocked by inhibiting its association with HSPG using heparin and binding was not observed in cells lacking surface HSPG. These results indicate that cell binding is critical for AU-011-mediated cytotoxicity. Although AU-011 can deliver hundreds of dye molecules, its tumor tropism was not affected by dye conjugation. Following intravenous administration in murine tumor models and intravitreal administration in rabbit uveal melanoma xenograft models, potent dose-dependent tumor response with histopathologically confirmed acute tumor cell necrosis was observed after photoactivation, and treatment spared the retina and adjacent ocular structures, showing its targeted effect.15,18
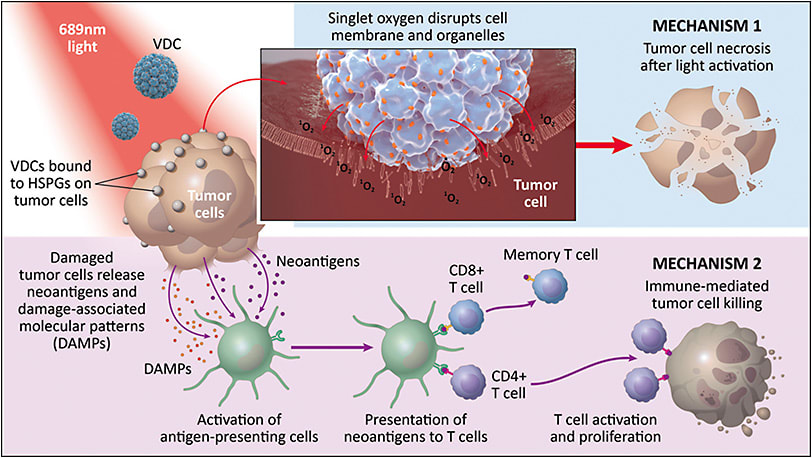
AU-011 has a novel dual mechanism of action (Figure 2). The first mechanism corresponds to acute tumor cell necrosis. AU-011 delivers hundreds of photosensitizer Irdye 700DX molecules that are promoted to the excited state on light activation and generate singlet oxygen. Singlet oxygen generation near the tumor cell membrane disrupts the membrane integrity of the tumor cell and causes acute tumor cell necrosis. In addition to acute tumor cell necrosis, AU-011’s activity leads to immune-mediated tumor cell killing, releasing tumor neoantigens that can activate CD-8 T cells and generate long-term antitumor immunity.19,20
SUPRACHOROIDAL VS INTRAVITREAL DELIVERY
In a rabbit uveal melanoma xenograft model, suprachoroidal administration of AU-011 showed 5 times higher tumor exposure compared to intravitreal injection.21 Mean tumor concentrations were 12,459±5,190 ng/mL in eyes with suprachoroidal injection vs 1,996±421 ng/mL in the eyes with intravitreal injection. There were negligible levels of AU-011 in the vitreous in the eyes with suprachoroidal injection. Immunohistochemical AU-011 staining was observed penetrating throughout the tumor in the suprachoroidal injected group, whereas AU-011 staining was mostly localized on the apex or vitreal surface of the tumor in the intravitreally injected group. This shows that suprachoroidal administration improved tumor distribution and bioavailability and has the potential to decrease side effects such as intraocular inflammation and vitreous floaters compared to intravitreal administration. Suprachoroidal delivery may optimize the treatment parameters by shortening the interval time to laser activation (4 to 6 hours with suprachoroidal vs 6 to 8 hours with intravitreal administration) and might increase the opportunity to treat additional patient populations such as medium-sized choroidal melanoma and choroidal metastasis.
CLINICAL TRIALS WITH AU-011 VIA SUPRACHOROIDAL ADMINISTRATION
AU-011 is currently being investigated in a phase 2 clinical trial comprised of an initial open-label dose-escalation phase to determine the safety and optimal treatment regimen followed by a randomized confirmatory phase to determine the safety and efficacy via suprachoroidal administration in subjects with primary indeterminate lesions and small choroidal melanoma.11 The initial dose-escalation phase will evaluate the safety and efficacy of single and multiple ascending doses of AU-011 via suprachoroidal administration, followed by 1 or 2 laser applications per treatment. Laser delivering light at 689 nm is applied over the choroidal tumor after 4 to 6 hours following the suprachoroidal injection of AU-011.
The open-label dose-escalation phase is currently ongoing, with 14 subjects enrolled.23 Cohorts 1 to 5 are fully enrolled and cohort 6 is enrolling now. The study started with the first cohort group receiving 20 µg of AU-011 followed by 1 laser administration. Based on the safety and tolerability to date, the dose of AU-011 was increased to 80 µg in 2 injections in separate quadrants followed by 2 laser administrations. The treatment is being repeated for up to 3 cycles, each cycle comprised of 3 weekly treatments of 80 µg dose and 2 laser administrations. No dose-limiting toxicities or treatment-related serious adverse events have been reported. The most common side effects related to AU-011 or laser were anterior chamber cell/inflammation (23%), followed by eye pain (15.4%), and punctate keratitis (15.4%). Most adverse effects were transient and resolved without clinical sequelae.
AU-011 FOR CHOROIDAL METASTASIS
The use of light-activated VDCs represents a novel approach for the first-line treatment of choroidal tumors. AU-011 is the first VDC in clinical development with a dual mechanism of action consisting of acute necrosis followed by an immune activation that may lead to long-term antitumor immunity. The tumor targeting is driven by the binding of the VDC to modified HSPGs on the tumor cell membrane. The favorable safety profile to date may lead to improved visual outcomes compared to the standard of care with radiotherapy, and suprachoroidal delivery may improve the therapeutic index and optimize treatment parameters compared to intravitreal administration. The preliminary safety data from the ongoing phase 2 trial using suprachoroidal administration supports the continued dose escalation to an 80 µg/day dose and up to 3 cycles of therapy.
CONCLUSION
Suprachoroidal administration has the potential to improve the therapeutic index of AU-011 for the treatment of patients with indeterminate lesions and small choroidal melanoma. Given the optimal benefit–risk profile, this novel approach could be used in many additional ocular oncology indications, such as choroidal metastasis or hemangioma. This novel route of administration could become a preferred route for treating retinal and choroidal diseases of the eye. Recently, the potential to treat choroidal metastasis of breast and lung cancer with AU-011 has been evaluated in vitro and in vivo. Rich et al noted that AU-011 can bind to, and kill, cells derived from the most common cancer types known to metastasize to the choroid, which supports further clinical development of AU-011.22 RP
Editor’s note: This article is part of a special edition of Retinal Physician that was supported by Bausch + Lomb.
REFERENCES
- The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma: COMS report no. 5. Arch Ophthalmol. 1997;115(12):1537-1544. doi:10.1001/archopht.1997.01100160707007
- Skinner CC, Augsburger JJ, Augsburger BD, Correa ZM. Comparison of alternative tumor size classifications for posterior uveal melanomas. Invest Ophthalmol Vis Sci. 2017;58(9):3335-3342. doi:10.1167/iovs.16-20465
- Singh AD, Grossniklaus HE. What’s in a name? Large choroidal nevus, small choroidal melanoma, or indeterminate melanocytic tumor. Ocul Oncol Pathol. 2021;7(4):235-238. doi:10.1159/000516536
- Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102(9):1351-1361.
- Shields CL, Cater J, Shields JA, Singh AD, Santos MC, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000;118(3):360-364. doi:10.1001/archopht.118.3.360
- Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127(8):981-987. doi:10.1001/archophthalmol.2009.151
- Butler P, Char DH, Zarbin M, Kroll S. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology. 1994;101(4):710-717. doi:10.1016/s0161-6420(94)31274-7
- Wen JC, Oliver SC, McCannel TA. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye (Lond). 2009;23(6):1254-1268. doi:10.1038/eye.2009.43
- Bianciotto C, Shields CL, Pirondini C, Mashayekhi A, Furuta M, Shields JA. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117(5):1005-1012. doi:10.1016/j.ophtha.2009.10.015
- Shields CL, Sioufi K, Srinivasan A, et al. Visual outcome and millimeter incremental risk of metastasis in 1780 patients with small choroidal melanoma managed by plaque radiotherapy [published correction appears in JAMA Ophthalmol. 2019 Jan 17]. JAMA Ophthalmol. 2018;136(12):1325-1333. doi:10.1001/jamaophthalmol.2018.3881
- Phase 2 trial to evaluate safety and efficacy of AU-011 via suprachoroidal administration in subjects with primary indeterminate lesions and small choroidal melanoma. ClinicalTrials.gov Identifier: NCT04417530. Updated February 25, 2022. Accessed February 28, 2022. https://clinicaltrials.gov/ct2/show/NCT04417530
- Study in subjects with small primary choroidal melanoma. ClinicalTrials.gov identifier: NCT03052127. Updated January 14, 2022. Accessed February 28, 2022. https://clinicaltrials.gov/ct2/show/NCT03052127
- Cho CF, Shukla S, Simpson EJ, Steinmetz NF, Luyt LG, Lewis JD. Molecular targeted viral nanoparticles as tools for imaging cancer. Methods Mol Biol. 2014;1108:211-230. doi:10.1007/978-1-62703-751-8_16
- Tornesello AL, Tagliamonte M, Buonaguro FM, Tornesello ML, Buonaguro L. Virus-like particles as preventive and therapeutic cancer vaccines. Vaccines (Basel). 2022;10(2):227. doi:10.3390/vaccines10020227
- Kines RC, Cerio RJ, Roberts JN, et al. Human papillomavirus capsids preferentially bind and infect tumor cells. Int J Cancer. 2016;138(4):901-911. doi:10.1002/ijc.29823
- Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83(5):2067-2074. doi:10.1128/JVI.02190-08
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106(48):20458-20463. doi:10.1073/pnas.0908502106
- Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8(6):10425-10436. doi:10.18632/oncotarget.14425
- Kines RC, Varsavsky I, Choudhary S, et al. An Infrared dye-conjugated virus-like particle for the treatment of primary uveal melanoma. Mol Cancer Ther. 2018;17(2):565-574. doi:10.1158/1535-7163.MCT-17-0953
- Kines RC, Thompson CD, Spring S, et al. Virus-like particle-drug conjugates induce protective, long-lasting adaptive antitumor immunity in the absence of specifically targeted tumor antigens. Cancer Immunol Res. 2021;9(6):693-706. doi:10.1158/2326-6066.CIR-19-0974
- Savinainen A, Grossniklaus HE, King S, Wicks J, Rich CC. Ocular distribution and exposure of AU-011 after suprachoroidal or intravitreal administration in an orthotopic rabbit model of human uveal melanoma. Invest Ophthalmol Vis Sci. 2021;62(8):2861.
- Rich CC, Savinainen A, Kines R. Development of AU-011 for choroidal metastasis. Invest Ophthalmol Vis Sci. 2021;62(8):44
- Demirci H. A phase 2 trial of a first in class targeted therapy for choroidal melanoma via suprachoroidal (SC) administration. Poster presented at the American Academy of Ophthalmology annual meeting, 2021, virtual.








