Over the last decade, intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents have been added to the armamentarium for treatment of diabetic retinopathy (DR), supplementing the preexisting therapeutic options of laser and vitrectomy. The FDA approved ranibizumab (Lucentis; Genentech) 0.3 mg in 2017 and aflibercept (Eylea; Regeneron) 2 mg in 2019 for treatment of DR without diabetic macular edema (DME), without specifying the level of DR severity. It is undisputed that treatment is necessary for eyes with proliferative diabetic retinopathy (PDR). The Diabetic Retinopathy Study demonstrated 4 decades ago that peripheral scatter laser treatment in PDR eyes featuring high-risk characteristics (Table 1) reduced the risk of severe vision loss (defined as visual acuity <5/200) by 50%. The standard of care has generally evolved to treat all PDR eyes without specifying high-risk features due to the increased risk for these eyes of progression to vision-threatening complications, and anti-VEGF injections have become a therapeutic option.1,2 However, there remains a lack of clarity regarding the use of intravitreal anti-VEGF injections in eyes with nonproliferative diabetic retinopathy (NPDR) without DME, as well as unanswered questions regarding anti-VEGF as the sole therapy for PDR eyes without DME. This article explores some of the recent studies that can be used by clinicians when making treatment decisions for eyes with diabetic retinopathy without center-involved DME.
| High-risk characteristics of proliferative diabetic retinopathy defined by the Diabetic Retinopathy Study report number 88 | Presence of neovascularization of the disc (NVD) equaling to or exceeding 1/3 to 1/4 disc area in extent, with or without vitreous hemorrhage or preretinal hemorrhage |
| Presence of vitreous hemorrhage or preretinal hemorrhage with less extensive new vessels, either NVD less than 1/3 to 1/4 disc area in extent or neovascularization elsewhere (NVE) of 1/2 disc area or more in extent | |
| High-risk characteristics of proliferative diabetic retinopathy defined at the time of the Diabetic Retinopathy Study 1976 protocol change18 | Moderate or severe NVE with vitreous or preretinal hemorrhage, or both |
| Mild NVD with vitreous or preretinal hemorrhage, or both | |
| Moderate or severe NVD with or without vitreous or preretinal hemorrhage, or both |
NONPROLIFERATIVE DIABETIC RETINOPATHY
For many retina specialists, there is a lack of clarity surrounding the utility of anti-VEGF for eyes with NPDR without DME. According to the 2021 Preferences and Trends (PAT) survey from the American Society of Retina Specialists, approximately 60% of US retina specialists closely monitor very severe NPDR and encourage systemic glycemic control, 25% consider anti-VEGF in some patients with poor sugar control, 8% consider anti-VEGF therapy in all or most patients, and 3% consider anti-VEGF therapy in some patients with good sugar control and compliance (4% of responders reported other).3
The PANORAMA trial was a recent multicenter, randomized, double-masked control trial that evaluated the efficacy and safety of aflibercept 2 mg for eyes with severe NPDR (defined as diabetic retinopathy severity score [DRSS] at level 47 or 53) without DME.4 In this trial, eyes were randomized to 1 of 3 groups: aflibercept 2 mg every 16 weeks for 100 weeks following 4 loading doses, aflibercept 2 mg every 8 weeks following 5 monthly doses to 52 weeks followed by as-needed (PRN) dosing in year 2, and sham injection as the control group. The primary endpoint was the proportion of eyes with a 2-step or greater improvement in DRSS level from baseline to week 24 and 52. The aflibercept group had a statistically significant reduction in the event rate for 2 or more step worsening in DRSS score as well as a significant reduction in risk of vision-threatening complications at weeks 52 and 100.
The results of the trial showed statistically significant improvement in 2-step or greater improvement in DRSS level from baseline in both of the aflibercept groups compared to the control group at week 24 and 100. The percentage of eyes with 2-step or greater improvement in DRSS improvement declined from 79% at week 52 to 50% at week 100 for eyes receiving PRN aflibercept in year 2, whereas the rates of vision-threatening complications increased, implying that some of the ant-VEGF benefits from aflibercept may not be sustained without regular injections. This is further supported by the fact that the DRSS improvements were maintained in the eyes that received aflibercept at ongoing, regular every-16-week intervals, including from week 52 to week 100. PANORAMA confirmed findings that were originally noted in the Early Treatment Diabetic Retinopathy Study (EDTRS): eyes with severe NPDR (defined as DRSS levels 47 or 53) have a very high risk of progressing to PDR, and this progression to PDR was seen as 30% in the PANORAMA control eyes at 2 years. Aflibercept reduced vision-threatening complications, defined as a new PDR event or new diabetic macular edema (DME) compared to control eyes.
A recent publication by Yonekawa et al highlighted the issues regarding anti-VEGF use for NPDR without DME.5 Although there is strong evidence that anti-VEGF reduces both the DRSS and the probability of developing PDR,4,6 the counterpoints against the use of anti-VEGF include that not all NPDR eyes progress to PDR without treatment, there are costs and risks of anti-VEGF injections, there are a lack of data regarding functional outcomes, and there are challenges with buy-in from patients, given that most NPDR patients without DME are largely asymptomatic.
Another concern expressed by some retina specialists is the lack of an endpoint once a physician has initiated anti-VEGF treatment for NPDR: Will these patients be committed to an indefinite course of anti-VEGF injections? Defining an endpoint for utilization of anti-VEGF for NPDR may improve guidelines for the long-term treatment plan. It has been demonstrated that systemic indicators of glycemic control, such as hemoglobin A1c, fasting blood sugar, and urine albumin to creatinine ratio correlate with the DR severity.7 It is possible that improvement of systemic diabetic indicators, such as hemoglobin A1c or microalbuminuria may indicate improved systemic control and function as a surrogate endpoint. There are some scenarios in which anti-VEGF treatment of NPDR may be considered, including for patients with poor outcomes related to laser or rapid disease progression in the contralateral eye and highly motivated patients, although some clinicians may also consider early PRP as well in these select patients.
PROLIFERATIVE DIABETIC RETINOPATHY
Although there is no debate regarding the need to treat PDR eyes, there is variability regarding the treatment modality with options including panretinal photocoagulation (PRP), anti-VEGF injection, pars plana vitrectomy (PPV) or a combination of therapies. The efficacy of PRP was demonstrated in the Diabetic Retinopathy Study8; however, there are recognized drawbacks, including decreased peripheral vision, difficulty with night vision, exacerbation or induction of DME, and continued disease progression in some eyes despite PRP.1
Anti-VEGF agents induce rapid regression of retinal neovascularization, at a much faster initial rate than PRP (Figure 1). However, anti-VEGF injections as the sole treatment for PDR without DME may still be approached with caution due to the concern for potential recurrence of retinal neovascularization and advancement of PDR associated with lapses in treatment. The DRCR Retina Network Protocol S was a multicenter randomized clinical trial that directly compared the use of PRP vs ranibizumab 0.5 mg for treatment of PDR with or without DME.1 Eyes randomized to ranibizumab 0.5 mg received monthly injections through 12 weeks, and treatment thereafter was dependent on the investigator’s assessment of neovascularization. Eyes with residual neovascularization received additional ranibizumab injection at week 16, at week 20, and then monthly starting at week 24 unless neovascularization had completely resolved or was stable following 2 consecutive injections. In the PRP group, 35% of eyes received ranibizumab at baseline for DME followed by an additional 18% within 2 years. For patients with baseline DME, there was an average of 5 ranibizumab injections for DME in year 1 and 9 within 2 years. For those without baseline DME there was an average of 3 injections prior to year 1 and 4 within 2 years. At 2 years, the mean visual acuity letter score improved by 2.8 in the ranibizumab group and 0.2 in the PRP group (P<.001) and the actual visual acuity letter score was 78.7 in the ranibizumab group and 76.2 in the PRP group, both of which are equivalent to Snellen 20/32. There was no significant difference in the percentage of eyes with 10-letter and 15-letter or more improvement or worsening between groups. There was a higher probability of developing DME by 2 years in the PRP group (28%) vs the ranibizumab group (9%) (P<.001). Vitreous hemorrhage, retinal detachments, and neovascular glaucoma were all higher in the PRP group. Injection-related endophthalmitis occurred in 1 eye in the ranibizumab group and 0 eyes in the PRP group.
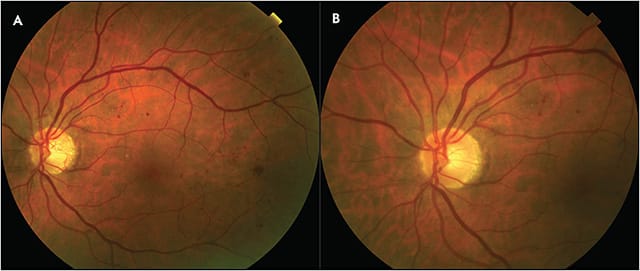
Ninety-seven percent of patients consented to continue in the study through 5 years.9 This consisted of study visits every 16 weeks in both groups, and injection frequency could range from every 4 weeks to every 16 weeks in the ranibizumab group. There was a large difference in the number of visits required between the 2 groups with an average of 43 visits in the ranibizumab group and 21 in the PRP group. In terms of visual outcomes at 5 years, both groups improved by a mean of 3 letters (P=.68) and achieved a mean Snellen visual acuity of 20/25. The rate of retinal detachment was higher in the PRP group (30 eyes vs 12 eyes), with similar numbers of vitreous hemorrhage and neovascular glaucoma. While initial enrollment in the 5-year study was high, only 61% of study eyes completed the study, demonstrating a high rate of loss to follow-up.
Many physicians are concerned about the implications of disruption in treatment if they are solely relying on anti-VEGF therapy without the security of PRP. Patients with diabetes are often medically complex, and hospitalizations can disrupt regularly scheduled visits. A 2019 study by Obeid et al evaluated the outcomes of patients lost to follow up (LTFU) for more than 6 months treated with either anti-VEGF agents or PRP. This study found statistically significant higher rates of tractional retinal detachments, neovascularization of the iris, and worsening of visual acuity (prior to LTFU visit Snellen 20/54, return visit 20/187, and last visit 20/166) in the anti-VEGF group.10 Although there was also statistically significant worsening of visual acuity in the PRP group at the return visit (prior to LTFU 20/53, return visit 20/83), there was no statistically significant difference compared to the final visit (20/58). These results highlight the higher frequency of adverse outcomes in real world scenarios if relying solely on pharmacotherapy to manage PDR. The risk of LTFU was further highlighted during the COVID-19 pandemic.11 According to the PAT survey in 2021, 74% of retina specialists reported that patients with DR were less likely to show up for appointments as a result of the pandemic.3
Although protocol S demonstrated noninferiority of anti-VEGF vs PRP in terms of visual outcomes in eyes with PDR, it would be prudent to exclude the difference in cost. Hutton et al evaluated such impacts by directly comparing the cost between patients with only 1 eye enrolled (213 participants) in the DRCR network Protocol S study over 2 years12 and found a $5,053 cost difference ($29,574 for ranibizumab, $24,520 for PRP) between the groups with DME at baseline and a $15,131 cost difference ($22,576 for ranibizumab, $7,445 for PRP) between the groups without DME at baseline. Although this may favor PRP in patients with PDR without DME, lower-cost anti-VEGF alternatives may present a more favorable cost profile.
Another concern with anti-VEGF use is the risk of crunch syndrome in PDR. While there are varying rates of anti-VEGF associated crunch syndrome in the literature ranging from 1.5% to 18.4%, a larger study by Arevalo et al assessing 698 eyes reported a rate of 3.6%.13,14 These patients had all received prior PRP and were treated with bevacizumab (Avastin; Genentech) due to refractory PDR. An earlier study also published by Arevalo et al found 27% of cases with crunch syndrome developed a new tractional retinal detachment (TRD), 64% had progression of an existing TRD, and 9% developed a combination TRD and rhegmatogenous RD occurring on average 13 days from the injection.15 As a result of these publications, a post hoc analysis with data pooled from 5 DRCR network studies was performed to assess the rate of crunch syndrome following anti-VEGF therapy.16 Eight hundred and eighty three eyes with PDR were included in the analysis, which revealed a 6.8% probability of developing a TRD in the first year of treatment with anti-VEGF compared to 4.5% in the control group. Interestingly, within the first 60 days of follow-up, the probability of developing a TRD was higher in the control eyes (2.6%) compared to anti-VEGF treated eyes (1.5%). Although the report by Bressler et al did not find an increased risk of crunch syndrome associated with anti-VEGF agents, it is still important to be mindful of this potential risk, specifically in patients with a preexisting TRD.
Another alternative to anti-VEGF is performing PPV. A study by Antoszyk et al compared aflibercept to vitrectomy with endolaser PRP for cases of vitreous hemorrhage from PDR.17 This randomized, multicenter, controlled trial included 205 eyes that were randomized to aflibercept or vitrectomy. The aflibercept group received aflibercept at baseline then every month for 3 months followed by injections at 16 weeks unless neovascularization was absent and 24 weeks unless there was stabilization. The vitrectomy group underwent a 23-gauge pars plana vitrectomy with complete PRP. Forty three percent of these patients received preoperative aflibercept, and patients could be treated with additional injections for recurrent hemorrhage 4 weeks after PPV. The results revealed faster visual acuity recovery in the vitrectomy group with a statistically significant difference in mean visual acuity letter score at 4 weeks but no statistically significant difference in mean visual acuity letter score at 24 weeks or at 2 years.
Given the controversial aspects of protocol S described earlier in this article, it is helpful to know the current practice patterns of retina specialists across the United States in terms of PDR management and specifically, the use of anti-VEGF. According to the 2021 PAT survey, the majority of retina specialists in the United States (69.9%) reported initiating anti-VEGF therapy with a plan for concurrent or future PRP in patients with high-risk PDR and center-involved DME, while only 4% reported treating with anti-VEGF alone.3
CONCLUSION
The traditional approach to DR has been reactive, but recent evidence of the effcacy of anti-VEGF injections for NPDR proposes a more proactive treatment approach. The lack of endpoint, concerns about adverse effects, and risk of poor adherence continue to serve as a barrier to initiating treatment in NPDR. Although the need for treatment of PDR is undisputed, relying solely on anti-VEGF treatment in eyes without DME remains controversial. While there is strong evidence of the noninferiority of anti-VEGF vs PRP for the management of PDR with good injection compliance, there are many barriers to regular treatment in this often medically and socially complex population that limits the utility of anti-VEGF as the sole treatment for PDR. RP
REFERENCES
- Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial [published correction appears in JAMA. 2016;315(9):944] [published correction appears in JAMA. 2019;321(10):1008]. JAMA. 2015;314(20):2137-2146. doi:10.1001/jama.2015.15217
- Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi:10.1016/S0140-6736(17)31193-5
- Hahn P. ASRS 2021 Preferences and Trends Membership Survey. Presented at: American Society of Retina Specialists Annual Meeting; 2021. Chicago, IL.
- Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946-955. doi:10.1001/jamaophthalmol.2021.2809
- Yonekawa Y, Modi YS, Kim LA, Skondra D, Kim JE, Wykoff CC. American Society of Retina Specialists clinical practice guidelines on the management of nonproliferative and proliferative diabetic retinopathy without diabetic macular edema. J Vitreoretin Dis. 2020;4(2):125-135. doi:10.1177/2474126419893829
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi:10.1016/j.ophtha.2011.12.039
- Hwang H, Kim JY, Oh TK, Chae JB, Kim DY. Relationship between clinical features of diabetic retinopathy and systemic factors in patients with newly diagnosed type II diabetes mellitus. J Korean Med Sci. 2020;35(23):e179. Published 2020 Jun 15. doi:10.3346/jkms.2020.35.e179
- Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583-600.
- Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial [published correction appears in JAMA Ophthalmol. 2019;137(4):467]. JAMA Ophthalmol. 2018;136(10):1138-1148. doi:10.1001/jamaophthalmol.2018.3255
- Obeid A, Su D, Patel SN, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology. 2019;126(3):407-413. doi:10.1016/j.ophtha.2018.07.027
- Ahmed I, Liu TYA. The impact of COVID-19 on diabetic retinopathy monitoring and treatment. Curr Diab Rep. 2021;21(10):40. doi:10.1007/s11892-021-01411-6
- Hutton DW, Stein JD, Glassman AR, et al. Five-year cost-effectiveness of intravitreous ranibizumab therapy vs panretinal photocoagulation for treating proliferative diabetic retinopathy: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2019;137(12):1424-1432. doi:10.1001/jamaophthalmol.2019.4284
- Tan Y, Fukutomi A, Sun MT, Durkin S, Gilhotra J, Chan WO. Anti-VEGF crunch syndrome in proliferative diabetic retinopathy: a review. Surv Ophthalmol. 2021;66(6):926-932. doi:10.1016/j.survophthal.2021.03.001
- Arevalo JF, Sanchez JG, Saldarriaga L, et al. Retinal detachment after bevacizumab. Ophthalmology. 2011;118(11):2304.e3-2304.e2.304E7. doi:10.1016/j.ophtha.2011.05.015
- Arevalo JF, Maia M, Flynn HW Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(2):213-216. doi:10.1136/bjo.2007.127142
- Bressler NM, Beaulieu WT, Bressler SB, et al. Anti-vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: pooled analysis of five DRCR retina network randomized clinical trials. Retina. 2020;40(6):1021-1028. doi:10.1097/IAE.0000000000002633
- Antoszyk AN, Glassman AR, Beaulieu WT, et al. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2020;324(23):2383-2395. doi:10.1001/jama.2020.23027
- Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85(1):82-106. doi:10.1016/s0161-6420(78)35693-1








