Susac syndrome is a rare disease that commonly affects females and young patients.1,2 It is an autoimmune vasculo-occlusive disease, in which the vascular endothelium is injured by circulating antibodies, and results in arterial microinfarcts affecting the central nervous system (CNS), retina, and inner ear.3-5
CRITERIA FOR DISEASE DIAGNOSIS
A study by the European Susac Consortium6 describes established criteria for diagnosis of either definite or probable Susac syndrome. In definite Susac, patients present an unequivocal clinical and/or paraclinical involvement of all 3 main organs (fulfilling the typical clinical triad). Cerebral involvement includes new cognitive impairment and/or behavioral changes and/or new focal neurological symptoms and/or new headache, and typical cranial MRI findings. The MRI findings include multiple small hyperintense foci on T2-weighted images, with contrast-enhancing lesions in white and gray matter both supratentorially and infratentorially and with corpus callosal and leptomeningeal involvement. These lesions can appear as “snowballs.” Callosal lesions involving the central fibers are considered pathognomic for Susac syndrome in the appropriate clinical setting, and occasional linear stranding from these areas may be seen.7 Retinal involvement includes branch retinal artery occlusion (BRAO) or arteriolar wall hyperfluorescence (AWH) or signs of branch retinal ischemia in fluorescein angiography (FA) (Figure 1). To note, the presence of AWH remote from retinal vascular injury may confirm diagnosis, because it has never been described in any other condition.8 Vestibulocochlear involvement includes new tinnitus and/or sensorineural hearing loss and/or peripheral vertigo, supported by objective testing.9
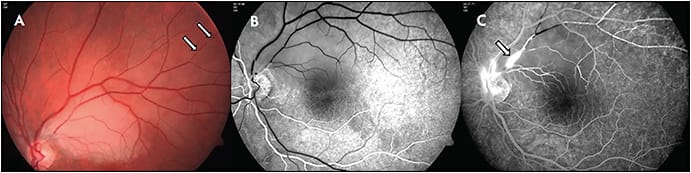
Probable Susac is defined as patients with an unequivocal clinical and/or paraclinical involvement of 2 of the 3 main organs. In patients with some of the typical features of Susac but who do not have involvement of 2 of the main organs, the diagnosis should be considered as possible, and these patients need careful and frequent follow-up because the diagnosis could evolve. Other uncommon systemic findings that may appear in Susac syndrome include skin involvement with livedo reticularis or livedo racemosa and arthralgias and myalgias.10
RETINAL FINDINGS
Signs of retinal involvement in Susac syndrome fall along a spectrum from symptomatic and funduscopically obvious BRAO to subtle asymptomatic peripheral involvement evident only on FA. Funduscopy may reveal Gass plaques, ie, yellow refractile lesions, simulating emboli. These are most probably caused by an immune-mediated localized reaction in the retinal arterial wall. In contrast to thrombin or cholesterol plaques, Gass plaques may be present in any arterial location and are not limited to the arteriolar bifurcation. In acute BRAO, typical sectoral whitening due to ischemia can be visible.10 Neovascularization and vitreous hemorrhage due to retinal ischemia are rare complications. In the later course of the disease, arterioarterial or arteriovenous collaterals may occur.11
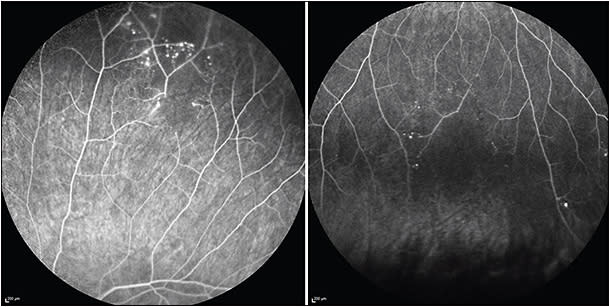
Visual field tests are a valuable tool to detect, quantify, and monitor visual field defects. These include altitudinal defects, paracentral scotoma, or central scotoma. Fundus FA is the diagnostic pillar in the workup for suspected Susac, as well as in the monitoring of treatment response and titration of therapy, even with normal funduscopy in asymptomatic patients. Susac syndrome may involve the retinal arteries from their large proximal branches up to small capillaries in the far periperhy. Fluorescein angiography can demonstrate BRAO already in the early frames, and BRAO can range from complete occlusion to only partial involvement. Arteriolar wall hyperfluorescence may accompany BRAO or be present in the absence of BRAO.12 Of note, AWH may not be evident in early photos but often becomes evident by mid and late frames. The extent of capillary nonperfusion becomes most clearly evident on late images. Recently, our group described microaneurysms as sequelae of peripheral ischemia in Susac syndrome (Figure 2).12 Accurate comparison between visits is of eminent importance in the monitoring of the disease. Therefore, all serial studies should to be done in exactly the same way (ie, showing the same areas at the same time). Also, the value of widefield FA has been shown lately, because peripheral findings might be missed with conventional FA.13
Optical coherence tomography (OCT) is a helpful tool to identify and monitor inner retinal ischemia at different stages, when located within the posterior pole. Like BRAO from other etiologies, acute retinal hypoxia presents with hyperreflective swelling of the inner retinal layers, and with inner retinal thinning during the later course. Optical coherence tomography angiography (OCTA) adds the ability to identify chronic microvascular changes. In areas of BRAO, OCTA demonstrates reduction of flow signals in the deep capillary plexus and less pronounced in the superficial capillary plexus.14 Even clinically unaffected Susac eyes have been shown to have poorer vascular parameters on OCTA.15 However, in cases of extramacular BRAO, vessel density maps of the central 3 mm might be normal. Compared to FA, OCTA is still inferior in the diagnosis of Susac syndrome, because OCTA is still limited in reliably displaying the retinal periphery due to limited resolution, motion artifacts, and difficult acquisition in patients with instable fixation or head motion due to severe neurological disease. Moreover, OCTA does not show vessel wall leakage (ie, AWH). The role of OCTA in the diagnostic algorithm of Susac syndrome is still under investigation.
TREATMENTS
Treatment of Susac syndrome is particularly challenging, owing to disease rarity and the great variability in presentation. In addition, there have been no randomized control trials or prospective treatment studies to evaluate treatment strategies. Optimal outcome requires rapid and aggressive treatment to achieve complete disease suppression. Acute treatments, limited to glucocorticoids and/or intravenous immunoglobulins, appear to be insufficient to halt the progression of disease.8,16-18 Rennebohm et al recently published treatment guidelines based on disease manifestations and severity.16 We established a multidisciplinary team at Tel Aviv Medical Center that includes a neurologist, rheumatologist, and an ophthalmologist. The team decides on treatment strategies and evaluates treatment response by continuing to monitor with MRI scans, audiometry, FA, OCT, and visual field tests. All patients are treated with high-dose corticosteroids (intravenous pulses of methylprednisolone over several days, and a long-term course of tapering oral steroids) combined with intravenous immune globulin. Adjunctive treatments for severe cases include cyclophosphamide or rituximab (Rituxan; Genentech) and mycofenolate mofetil (Cellcept; Genentech) for mild to moderate cases. These treatments are given for long periods.
DISEASE OUTCOME
Outcome may variate between patients. Some patients may present with a mild, relatively brief disease and experience mild or reversible ischemic dysfunction in the brain, retina, and/or inner ear with little or no residual damage. At the other end are patients with severe, prolonged disease (lasting several years) who sustain devastating permanent damage.
SUMMARY
Susac syndrome may be underdiagnosed because clinical manifestations may vary between patients and may resemble other neurologic, ophthalmic, and auditory diseases. A high index of suspicion for disease occurrence should alert the treating physician (neurologists, ophthalmologists, ENT specialists) to refer the patient for a thorough neurologic, ocular, and sensorineural evaluation. In recent years, the incidence of Susac patients in our medical center (Tel Aviv Medical Center) significantly increased compared to previous years and to the expected incidence according to population size.19 This may be explained by physicians’ awareness of the disease and of a good multidisciplinary collaboration as part of the disease workup. In addition, the duration from symptom onset to disease diagnosis has significantly shortened, allowing prompt diagnosis and initiation of appropriate treatment, which may result in an improved disease outcome. It is crucial to adequately monitor patients and adjust the treatment according to disease activity. RP
REFERENCES
- Dörr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9(6):307-316. doi:10.1038/nrneurol.2013.82
- Seifert-Held T, Langner-Wegscheider BJ, Komposch M, et al. Susac syndrome: clinical course and epidemiology in a Central European population. Int J Neurosci. 2017;127(9):776-780. doi:10.1080/00207454.2016.1254631
- Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316. doi:10.1212/wnl.29.3.313
- Susac JO. Susac syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593. doi:10.1212/wnl.44.4.591
- Magro CM, Poe JC, Lubow M, Susac JO. Susac syndrome: an organ-specific autoimmune endotheliopathy syndrome associated with anti-endothelial cell antibodies. Am J Clin Pathol. 2011;136(6):903-912. doi:10.1309/AJCPERI7LC4VNFYK
- Kleffner I, Dörr J, Ringelstein M, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry. 2016;87(12):1287-1295. doi:10.1136/jnnp-2016-314295
- Gross M, Eliashar R. Update on Susac syndrome. Curr Opin Neurol. 2005;18(3):311-314. doi:10.1097/01.WCO.0000169751.46568.F0
- Egan RA. Diagnostic criteria and treatment algorithm for Susac syndrome. J Neuroophthalmol. 2019;39(1):60-67. doi:10.1097/WNO.0000000000000677
- Oron Y, Handzel O, Habot-Wilner Z, et al. Vestibular function assessment of Susac syndrome patients by the video head impulse test and cervical vestibular-evoked myogenic potentials. J Vestib Res. 2020;30(6):393-399. doi:10.3233/VES-200007
- Agarwal A. Macular dysfunction caused by retinal vascular diseases. In: Agarwal A, ed. Gass’ Atlas of Macular Diseases. 5th ed. Elsevier, Saunders; 2012:596-601.
- Egan RA, Jirawuthiworavong G, Lincoff NS, Chen JJ, Francis CE, Leavitt JA. Retinal arterio-arterial collaterals in Susac syndrome. J Neuroophthalmol. 2018;38(4):459-461. doi:10.1097/WNO.0000000000000627
- Zur D, Goldstein M, Barequet D, et al. Susac’s syndrome — a new ocular finding and disease outcome. Eye (Lond). 2022;36(4):781-788. doi:10.1038/s41433-021-01464-7
- Turczyńska MJ, Krajewski P, Brydak-Godowska JE. Widefield fluorescein angiography in the diagnosis of Susac syndrome. Retina. 2021;41(7):1553-1561. doi:10.1097/IAE.0000000000003051
- Mastropasqua R, Toto L, Senatore A, et al. Optical coherence tomography angiography findings in Susac’s syndrome: a case report. Int Ophthalmol. 2018;38(4):1803-1808. doi:10.1007/s10792-017-0653-9
- Wirth MA, Khan HM, Chan J, et al. Investigating microangiopathy using swept-source optical coherence tomography angiography in patients with Susac syndrome. Retina. 2021;41(10):2172-2178. doi:10.1097/IAE.0000000000003170
- Rennebohm RM, Asdaghi N, Srivastava S, Gertner E. Guidelines for treatment of Susac syndrome — an update. Int J Stroke. 2020;15(5):484-494. doi:10.1177/1747493017751737
- Dörr J, Ringelstein M, Duning T, Kleffner I. Update on Susac syndrome: new insights in brain and retinal imaging and treatment options. J Alzheimers Dis. 2014;42 Suppl 3:S99-S108. doi:10.3233/JAD-132519
- Capiluppi E, Romano L, Luccarelli SV, Macerollo A, Cislaghi G. Aggressive immunosuppression in Susac syndrome: 10 years of follow-up. Neurol Sci. 2018;39(10):1807-1809. doi:10.1007/S10072-018-3481-4
- Wilf-Yarkoni A, Elkayam O, Aizenstein O, et al. Increased incidence of Susac syndrome: a case series study. BMC Neurol. 2020;20(1). doi:10.1186/S12883-020-01892-0








