Multiple evanescent white dot syndrome (MEWDS) belongs to the family of white dot syndromes. It is an idiopathic inflammatory chorioretinopathy that resolves without treatment.1 MEWDS typically presents in healthy young female patients (with a female-to-male ratio of 5:1) between the second and fourth decades of life as acute, unilateral lesions preceded by a flu-like illness. The usual presenting symptoms are blurring of vision, photopsia, floaters, dyschromatopsia, temporal visual field loss, and paracentral or temporal scotomas.1-3
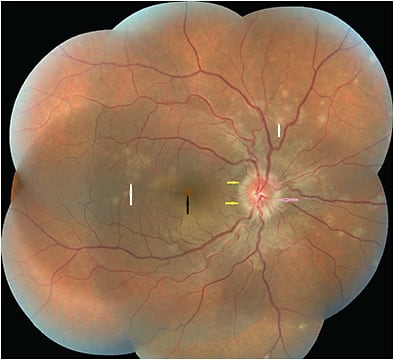
Small greyish-white lesions are visible the level of the retinal pigment epithelium (RPE), outer retina, and inner choroid, extending from the posterior pole to the equator with maximum concentration at the perimacular area. Lesions may coalesce around the optic disc and along the vascular arcades. Granularity at the fovea is the most characteristic finding. MEWDS can be accompanied by a relative afferent pupillary defect, posterior vitreous cells, and optic disc edema.1-3
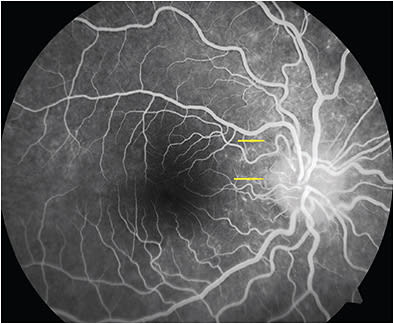
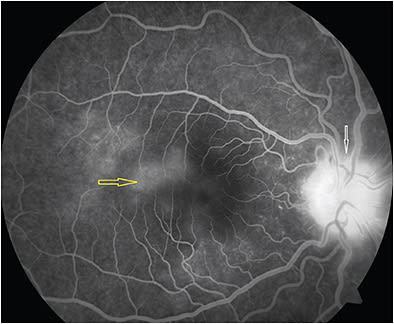
Recent reports describe 2 types of lesions based on fluorescein angiography (FA) and indocyanine green angiography (ICGA): spots, about 200 µm in diameter at the RPE/photoreceptor junction in the midperipheral retina, and dots, <100 µm in diameter in the outer nuclear layer, predominantly around the optic nerve head and nasal retina that may align below the retinal vessels causing venous sheathing. Both spots and dots are absent at the fovea (Figures 1-3).4-6
PATHOGENESIS
MEWDS does not have any known racial or hereditary predilection7; however, reports have shown a threefold increase in HLA-B51 association in persons affected by MEWDS.8 MEWDS has been shown to develop following Epstein-Barr virus infection,9 hepatitis A,10 hepatitis B vaccination,11 and human papilloma virus vaccination.12 Recurrent MEWDS has been linked to Herpesviridae infection or virus-induced autoimmune inflammatory reaction.13 The proposed mechanism is that MEWDS is triggered by an autoimmune response following infection with viruses similar to what has been noted in acute zonal occult outer retinopathy (AZOOR) complex disorders.14 Another hypothesis is that inflammation and autoimmune diseases are discrete entities and their presentation is based on the interaction of genetics, immune system pathways, and environmental triggers (eg, viral infection, immunization, stress, female sex).15 Recent evidence suggests that inflammation is at the level of the outer photoreceptor layer causing “photoreceptoritis,” and loss of inner and outer segments, and that it recovers in most cases.16
MULTIMODAL IMAGING
Fluorescein Angiography
Fluorescein angiography reveals early punctate hyperfluorescence in a wreath-like pattern with late staining and may show vascular and disc leakage. This is believed to be due to RPE window defects, intraretinal microangiopathy following inflammation, or both.7 Excitation of microglia due to inflammation and the disease’s occurrence in a stellate configuration have been implicated in the wreath-like pattern on FA.5 Both dots and spots show early hyperfluorescence, and most dots appear during the choroidal filling phase, whereas some become apparent during the retinal artery perfusion phase. Fluorescein angiography resolves with the resolution of the lesions.4 Fluorescein angiography within 1 week of onset of symptoms of scotoma, photopsia, and minimal loss of vision (the hyper early stage) shows a hypofluorescent perifoveolar halo surrounded by faint hyperfluorescence in the intermediate and late phases along with classical features of MEWDS.17
Indocyanine Green Angiography
Indocyanine green angiography (ICGA) shows hypocyanescence of dots and spots and peripapillary hypocyanescence associated with an enlarged blind spot on visual field testing. The lesions may surpass those seen clinically or evident on FA. They can be observed during the early, intermediate, or late phase and even in the fellow eye.5,7 Hypocyanescence of lesions has been attributed to the inflammatory deposits affecting the outer blood–retinal barrier, choriocapillaris flow impairment due to shunting caused by inflammation, low perfusion pressure or occlusion, poor affinity for ICG by the inflammatory debris, or reduced uptake by the altered RPE.7 In the hyper early stage, late-phase ICGA shows a hyperfluorescent perifoveolar halo and cockade-like appearance along with the classical features of hypofluorescent spots and dots.17
Fundus Autofluorescence
Fundus autofluorescence (FAF) shows hyperautofluorescence corresponding to the spots and dots.7 The hyperautofluorescence is believed to be due to choroidal inflammation leading to increased phagocytosis of photoreceptor outer segments with increased production of lipofuscin granules. The blue FAF (excitation wavelength of 488 nm) shows hypoautofluorescence of the dots at the fovea and hyperautofluorescence of the spots in the rest of the fundus that correspond to the spots seen on FA and ICGA and disruption of the ellipsoid zone (EZ) on optical coherence tomography (OCT). Near-infrared FAF (excitation wavelength of 787 nm) shows hypoautofluorescence of foveal lesions as well as dots and spots that corresponded clinically and by ICGA. The difference in fluorescence is due to the hypermetabolic RPE and accumulation of lipofuscin in blue autofluorescence and severely damaged RPE with melanin involvement in near-infrared autofluorescence and due to the difference in the metabolic pathway of both the pigments. Near-infrared FAF has shown superiority over blue FAF in identifying all lesions, including those at the fovea and those that are consistent with ICGA findings.5,18,19
Ultrawidefield FAF demonstrates hyperautofluorescence in the peripapillary area (corresponds as enlarged blind spot in the visual fields) and posterior pole in the acute stage (within 3 weeks after onset of MEWDS) and spreads centrifugally. Peripheral hyperautofluorescent spots are shown to disappear first with persistence of hyperautofluorescent spots at the posterior pole in the intermediate stage (between 3 and 8 weeks). In the late stage (beyond 9 weeks), most of the posterior pole hyperautofluorescent lesions return to normalcy and only a few of them show faint granular hyperautofluorescent lesions at the posterior pole. Thus, the hyperautofluorescent lesions spread in a centrifugal manner from the peripapillary area and disappear in a centripetal manner during resolution of MEWDS.20,21
Fundus autofluorescence can also reveal white dots as multiple hyperautofluorescent lesions surrounding the posterior pole in cases where the clinical presentation is consistent with MEWDS, presenting as foveal granularity (seen as attenuation of photoreceptor inner segment/outer segment [IS/OS] junction in spectral-domain OCT) but with no clear white dots or hypopigmented lesions on fundus examination.22
Optical Coherence Tomography
Dome-shaped hyperreflective lesions in the region of dots in the subretinal space are seen on OCT, along with attenuation and disruption of the IS/OS junction. Increased reflectivity of the choroid beneath the lesions has been observed and is due to inflammatory aggregates. The hyperreflective lesions correlated with the subretinal dots resolve, and the IS/OS junction slowly is restored.7,23 The photoreceptor cell bodies are always intact, permitting complete recovery of the photoreceptor outer segments.16 In recurrent MEWDS, the outer nuclear layer (ONL) thins, suggesting that recurrent MEWDS can lead to photoreceptor atrophy.24
Spectral-domain OCT shows dome-shaped hyperreflective material on the RPE extending to the inner retina through disruption of the EZ, interdigitation zone (IZ), and ONL (Figure 4).6 Irregularities of the RPE have been reported. Extrafoveal lesions corresponding to dots and spots show disruption of the EZ and IZ. Peripapillary fluid can be seen as a hyporeflective accumulation or as a pigment epithelial detachment on OCT that can progress to peripapillary atrophy following resolution of MEWDS.19,25 In the hyper early stage of MEWDS, the RPE layer is shown to be split with intact EZ, IZ, and external limiting membrane (ELM) in the perimacular area. The ellipsoid zone is shown to be disrupted in the peripapillary zone corresponding to hyperautofluorescent lesions on OCT. Spectral-domain OCT shows subfoveal hyperreflective lesion with disruption of EZ, IZ, and ELM. All of these changes are shown to resolve with disease resolution.17
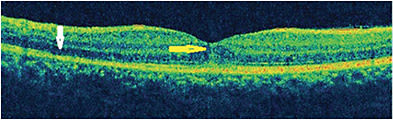
En face OCT reveals disruption of the EZ and IZ corresponding to the spots and dots, hyperreflective material in the outer portion of the ONL corresponding to the dots, and hyporeflective spots that are localized to the EZ. The choriocapillaris, choroid, and RPE, however, remain unaffected.16
Enhanced-depth imaging OCT shows transient outer retinal layer changes in the subfoveal and extrafoveal areas, along with a focal change in reflectivity of EZ and ELM that disappears during the recovery phase. Discrete hyperreflective dots are seen at the inner choroid (Sattler-choriocapillaris level) below the RPE, along with focal outer retinal layer changes in 40% of cases. It is postulated that these hyperreflective dots at the level of inner choroid could be due to deposition of inflammatory cells following focal inflammatory damage to choriocapillaris or pigment accumulation in the choroidal stroma. Increase in choroidal thickness is seen not only in the subfoveal area but outside the subfoveal area with involvement of the entire choroid (involving both the Sattler-choriocapillaris layer and the large choroidal vessel layer) in the acute stage that completely resolves with the resolution of the disease. This is speculated to be due to the inflammation spreading from the inner choroid to the entire choroid because of the anastomotic connections between medium and large choroidal vessels.26
Optical Coherence Tomography Angiography
In MEWDS, blood flow in the superficial and deep retinal capillaries, the choroidal vasculature, and in inner choroid, as well as the size of the foveal avascular zone, are unremarkable. This indicates preservation of blood flow in the retinal vasculature, choriocapillaris, and choroid.16,27
Visual Fields and Electrophysiology
Visual field testing demonstrates an enlarged blind spot. Central or paracentral scotomas may be present.7 The acute stage of MEWDS shows reduced a-wave amplitude on Ganzfield electroretinogram (impaired photoreceptor function) and reduced early receptor potential amplitudes and prolongation of its regeneration kinetics (visual pigment optical density of the outer segments), which recovers completely with the resolution of the condition.28 Multifocal electroretinogram shows supernormal N1 and P1 amplitudes at the early stage (day 1-7 after the start of the disease), which decreases by 2 weeks to return to normal or remain decreased after 2 weeks with no change in latency.29
Electro-oculogram shows a decrease in Arden ratio, which recovers completely.2,3 Electro-oculogram shows reduced Arden ratio in bilateral disease and in the eye with severe involvement, suggesting RPE involvement in the acute stage that resolves without sequelae.3,30
Foveal Reflection Analysis
The Stiles–Crawford effect denotes the direction of normal healthy cones toward the center of the pupil and has been studied by foveal reflection analysis. In MEWDS, foveal reflection analysis has demonstrated temporary disarray of cones, which regain their orientation during the recovery phase.31
OTHER ASSOCIATIONS
MEWDS has reportedly been associated with, preceded by, or followed by acute macular neuroretinopathy, AZOOR, multifocal choroiditis, and panuveitis.32-35 It may involve both eyes, recur, and be associated with choroidal neovascular membrane or chorioretinal scars as late complications.30,36-38
SUMMARY
MEWDS is generally self-limiting and presents as an acute unilateral condition predominantly in females, usually following a flu-like illness. Lesions can be spots at the level of the RPE/photoreceptor junction and dots at the level of the ONL with classical granular pigmentation at the fovea. There may be optic nerve head edema, vascular sheathing, or a relative afferent pupil defect associated with MEWDS. Fluorescein angiography findings include early punctate hyperfluorescence in a wreath-like pattern and late staining. Indocyanine green angiography shows hypocyanescence of both spots and dots and peripapillary hypocyanescence that correlates with an enlarged blind spot on visual field testing. Additional lesions not seen clinically or on FA can be seen with ICGA. Blue FAF shows hyperautofluoresence of the spots as seen in FA, ICGA, and EZ disruption on OCT and hypoautofluorescence of the foveal lesions, while near-infrared autofluorescence shows hypoautofluorescence corresponding to the spots and dots on ICGA both in the fovea and the rest of the fundus. On OCT, hyperreflective material on the RPE extending into the inner retina and disruption of the EZ and IZ can be seen. The photoreceptor cell bodies remain intact, which suggests that the photoreceptor IS and OS recover in the majority of cases. MEWDS is a disease of the outer retina with preservation of blood flow (as seen on OCTA) in the choriocapillaris, choroid, and retinal vasculature. It typically carries a good prognosis and resolves within weeks to months, although it may recur and may be associated with late complications, such as choroidal neovascular membrane and chorioretinal scar formation. RP
REFERENCES
- Jampol LM, Sieving PA, Pugh D, Fishman GA, Gilbert H. Multiple evanescent white dot syndrome. I. Clinical findings. Arch Ophthalmol. 1984;102(5):671-674. doi:10.1001/archopht.1984.01040030527008
- Polk TD, Goldman EJ. White-dot chorioretinal inflammatory syndromes. Int Ophthalmol Clin. 1999;39(4):33-53. doi:10.1097/00004397-199903940-00005
- Raven ML, Ringeisen AL, Yonekawa Y, Stem MS, Faia LJ, Gottlieb JL. Multi-modal imaging and anatomic classification of the white dot syndromes. Int J Retina Vitreous. 2017;3:12. Published 2017 Mar 20. doi:10.1186/s40942-017-0069-8
- Gross NE, Yannuzzi LA, Freund KB, Spaide RF, Amato GP, Sigal R. Multiple evanescent white dot syndrome. Arch Ophthalmol. 2006;124(4):493-500. doi:10.1001/archopht.124.4.493
- Tavallali A, Yannuzzi LA. MEWDS, common cold of the retina. J Ophthalmic Vis Res. 2017;12(2):132-134. doi:10.4103/jovr.jovr_241_16
- Chandrasekaran PR. Fundus fluorescein angiography and optical coherence tomographic characteristics in acute multiple evanescent white dot syndrome. Kerala J Ophthalmol. 2021;33(2):214-216. DOI:10.4103/kjo.kjo_14_21
- dell’Omo R, Pavesio CE. Multiple evanescent white dot syndrome (MEWDS). Int Ophthalmol Clin. 2012;52(4):221-228. doi:10.1097/IIO.0b013e31826647ed
- Borruat FX, Herbort CP, Spertini F, Desarnaulds AB. HLA typing in patients with multiple evanescent white dot syndrome (MEWDS). Ocul Immunol Inflamm. 1998;6(1):39-41. doi:10.1076/ocii.6.1.39.8084
- Yang CS, Hsieh MH, Su HI, Kuo YS. Multiple evanescent white dot syndrome following acute Epstein-Barr virus infection. Ocul Immunol Inflamm. 2019;27(2):244-250. doi:10.1080/09273948.2017.1371763
- Fine L, Fine A, Cunningham ET Jr. Multiple evanescent white dot syndrome following hepatitis a vaccination. Arch Ophthalmol. 2001;119(12):1856-1858. doi:10.1001/archopht.119.12.1870
- Baglivo E, Safran AB, Borruat FX. Multiple evanescent white dot syndrome after hepatitis B vaccine. Am J Ophthalmol. 1996;122(3):431-432. doi:10.1016/s0002-9394(14)72074-4
- Ogino K, Kishi S, Yoshimura N. Multiple evanescent white dot syndrome after human papillomavirus vaccination. Case Rep Ophthalmol. 2014;5(1):38-43. Published 2014 Feb 1. doi:10.1159/000358870
- Haw YL, Yu TC, Yang CS. A CARE-compliant article: a case report of possible association between recurrence of multiple evanescent white dot syndrome and the Herpesviridae family. Medicine (Baltimore). 2020;99(15):e19794. doi:10.1097/MD.0000000000019794
- Gass JD. Are acute zonal occult outer retinopathy and the white spot syndromes (AZOOR complex) specific autoimmune diseases?. Am J Ophthalmol. 2003;135(3):380-381. doi:10.1016/s0002-9394(03)00030-8
- Jampol LM, Becker KG. White spot syndromes of the retina: a hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am J Ophthalmol. 2003;135(3):376-379. doi:10.1016/s0002-9394(02)02088-3
- Pichi F, Srvivastava SK, Chexal S, et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: new insights into pathogenesis. Retina. 2016;36 Suppl 1:S178-S188. doi:10.1097/IAE.0000000000001255
- Cahuzac A, Wolff B, Mathis T, Errera MH, Sahel JA, Mauget-Faÿsse M. Multimodal imaging findings in ‘hyper-early’ stage MEWDS. Br J Ophthalmol. 2017;101(10):1381-1385. doi:10.1136/bjophthalmol-2016-309175
- Hua R, Chen K, Liu LM, Liu NN, Chen L, Teng WP. Multi-modality imaging on multiple evanescent white dot syndrome-A Spectralis Study. Int J Ophthalmol. 2012;5(5):644-647. doi:10.3980/j.issn.2222-3959.2012.05.21
- Marsiglia M, Gallego-Pinazo R, Cunha de Souza E, et al. Expanded clinical spectrum of multiple evanescent white dot syndrome with multimodal imaging. Retina. 2016;36(1):64-74. doi:10.1097/IAE.0000000000000685
- Hashimoto H, Kishi S. Ultra-wide-field fundus autofluorescence in multiple evanescent white dot syndrome. Am J Ophthalmol. 2015;159(4):698-706. doi:10.1016/j.ajo.2015.01.015
- Battaglia Parodi M, Iacono P, Falcomatà B, Bolognesi G, Bandello F. Near-infrared fundus autofluorescence in multiple evanescent white-dot syndrome. Eur J Ophthalmol. 2015;25(1):43-46. doi:10.5301/ejo.5000534
- Joseph A, Rahimy E, Freund KB, Sarraf D. Fundus autofluorescence findings and ancillary imaging in MEWDS patients without white dots. Invest Ophthalmol Vis Sci. 2013;54:3592.
- Amin HI. Optical coherence tomography findings in multiple evanescent white dot syndrome. Retina. 2006;26(4):483-484. doi:10.1097/01.iae.0000238558.14531.78
- Nguyen MH, Witkin AJ, Reichel E, et al. Microstructural abnormalities in MEWDS demonstrated by ultrahigh resolution optical coherence tomography. Retina. 2007;27(4):414-418. doi:10.1097/01.iae.0000246676.88033.25
- Sheng Y, Sun W, Gu YS. Spectral-domain optical coherence tomography dynamic changes and steroid response in multiple evanescent white dot syndrome. Int J Ophthalmol. 2017;10(8):1331-1333. Published 2017 Aug 18. doi:10.18240/ijo.2017.08.24
- Fiore T, Iaccheri B, Cerquaglia A, et al. Outer Retinal and Choroidal Evaluation in Multiple Evanescent White Dot Syndrome (MEWDS): An Enhanced Depth Imaging Optical Coherence Tomography Study. Ocul Immunol Inflamm. 2018;26(3):428-434. doi:10.1080/09273948.2016.1231329
- Yannuzzi NA, Swaminathan SS, Zheng F, et al. Swept-source OCT angiography shows sparing of the choriocapillaris in multiple evanescent white dot syndrome. Ophthalmic Surg Lasers Imaging Retina. 2017;48(1):69-74. doi:10.3928/23258160-20161219-10
- Sieving PA, Fishman GA, Jampol LM, Pugh D. Multiple evanescent white dot syndrome. II. Electrophysiology of the photoreceptors during retinal pigment epithelial disease. Arch Ophthalmol. 1984;102(5):675–9.
- Feigl B, Haas A, El-Shabrawi Y. Multifocal ERG in multiple evanescent white dot syndrome. Graefes Arch Clin Exp Ophthalmol. 2002;240(8):615-621. doi:10.1007/s00417-002-0478-7
- Aaberg TM, Campo RV, Joffe L. Recurrences and bilaterality in the multiple evanescent white-dot syndrome. Am J Ophthalmol. 1985;100(1):29-37. doi:10.1016/s0002-9394(14)74979-7
- Kanis MJ, van Norren D. Integrity of foveal cones in multiple evanescent white dot syndrome assessed with OCT and foveal reflection analyser. Br J Ophthalmol. 2006;90(6):795-796. doi:10.1136/bjo.2005.088484
- Gass JD, Hamed LM. Acute macular neuroretinopathy and multiple evanescent white dot syndrome occurring in the same patients. Arch Ophthalmol. 1989;107(2):189-193. doi:10.1001/archopht.1989.01070010195021
- Callanan D, Gass JD. Multifocal choroiditis and choroidal neovascularization associated with the multiple evanescent white dot and acute idiopathic blind spot enlargement syndrome. Ophthalmology. 1992;99(11):1678-1685. doi:10.1016/s0161-6420(92)31755-5
- Bryan RG, Freund KB, Yannuzzi LA, Spaide RF, Huang SJ, Costa DL. Multiple evanescent white dot syndrome in patients with multifocal choroiditis. Retina. 2002;22(3):317-322. doi:10.1097/00006982-200206000-00010
- Fine HF, Spaide RF, Ryan EH Jr, Matsumoto Y, Yannuzzi LA. Acute zonal occult outer retinopathy in patients with multiple evanescent white dot syndrome. Arch Ophthalmol. 2009;127(1):66-70. doi:10.1001/archophthalmol.2008.530
- Tsai L, Jampol LM, Pollock SC, Olk J. Chronic recurrent multiple evanescent white dot syndrome. Retina. 1994;14(2):160-163. doi:10.1097/00006982-199414020-00009
- Kozielec GF, Wyhinny GJ, Jampol LM. Evolution of distinct chorioretinal scars in recurrent MEWDS. Retina. 2001;21(2):180-182. doi:10.1097/00006982-200104000-00016
- Oh KT, Christmas NJ, Russell SR. Late recurrence and choroidal neovascularization in multiple evanescent white dot syndrome. Retina. 2001;21(2):182-184. doi:10.1097/00006982-200104000-00017








