An aging population and widespread use of antithrombotic (AT) agents for comorbid conditions presents the dilemma for retinal surgeons of having to perform surgery on patients receiving these agents. Oh et al reported that 14.6%, 28.5%, and 32.3% of patients undergoing vitreoretinal surgery were on antiplatelet (AP) agents in 1993, 2004, and 2008, respectively.1 Mason et al found warfarin or clopidogrel use in 179 of 289 (62%) consecutive patients undergoing 25-gauge pars plana vitrectomy.2
Retinal surgeon should carefully consider the risks and benefits of either continuing or stopping antithrombotic agents in consultation with patients and their medical providers, because either choice can have serious consequences. For example, an Ophthalmic Mutual Insurance Company (OMIC) closed-claim case study involving a routine vitrectomy for asteroid hyalosis on a patient receiving aspirin and clopidogrel dual antiplatelet therapy (DAPT) that resulted in loss of the eye due to intractable intraoperative bleeding resulted in a $825,000 settlement.3 On the other hand, injudicious and indiscriminate discontinuation of AT agents can result in serious systemic events such as heart attack, stroke, or death.
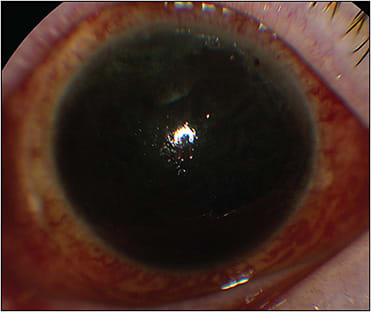
Because a patient on antithrombotics is at high risk from both ocular and systemic perspectives (Figures 1 and 2), it is important for a retina surgeon to have some working knowledge of the indications, management strategies, and pharmacology of commonly used antithrombotic agents. This review aims to provide retina surgeons knowledge and insight enabling them to effectively communicate these complex issues with patients and their medical providers to strive for the best possible outcomes for each patient.

OVERVIEW OF ANTIPLATELET AGENTS AND ANTICOAGULANTS
Antiplatelet Agents
Antiplatelet agents inhibit platelet activation. Current oral AP agents belong to 3 pharmacologic classes: thromboxane A2 inhibitor (aspirin), thienopyridines (clopidogrel, prasugrel) and cyclopentyltriazolopyrimidines (ticagrelor [Brilinta; AstraZeneca]) (Table 1).4 Aspirin inhibits platelet activation by irreversibly binding the enzyme cyclooxygenase-1, thereby preventing arachidonic acid from metabolizing into thromboxane A2 (TXA2), a potent mediator of platelet activation and vasoconstrictor. Aspirin is rapidly absorbed after oral ingestion and is cleared from the plasma within 1 hour. Platelets formed thereafter are not affected. Ten percent to 15% of platelet population is replenished daily. Aspirin is a relatively weak AP agent because it only partially inhibits TXA2 synthesis; thus, other non-TXA2 dependent activators, such as adenosine diphosphate (ADP), collagen, and thrombin, can bypass the inhibitory effect of aspirin. Spontaneous recovery of platelet function and in vitro studies have shown that only 30% to 40% of native platelets, corresponding to 3 to 4 days of aspirin interruption, are adequate to produce sufficient TXA2 to achieve a normal activation and aggregation response.5 Therefore, it is unnecessary, and potentially harmful, to interrupt aspirin therapy beyond 3 to 5 days prior to surgery.6
| DRUG/CLASS | MECHANISM OF ACTION | HALF-LIFE | COMMON INDICATIONS | INTERRUPTION PERIOD |
| Aspirin | Irreversible inhibition of COX-1 | 20 minutes | Primary prevention of ASCVD, CAD, secondary prevention of ACS, AIS, PAD | 3-5 days (adequate recovery) |
| Clopidogrel | Irreversible blockade of P2Y12 receptor by active metabolite (2-step process) | 6 hours for prodrug, 8 hours for inactive metabolite | DAPT, secondary prevention of ACS, AIS, PAD | 5-7 days |
| Ticagrelor | Direct-acting reversible, antagonist of P2Y12 receptor | 8-12 hours | DAPT, secondary prevention of ACS, AIS, PAD | 3-5 days |
| Prasugrel | Irreversible inhibition of P2Y12 receptor by active metabolite (1-step process) | 30-60 minutes (active metabolite) | DAPT after PCI | 7-10 days |
| Warfarin | Vitamin K antagonist | Variable | AF, mechanical heart valve, VTE, PE | 3-5 days |
| Dabigatran | Direct thrombin inhibitor | 12-14 hours | AF, VTE, VTE after surgery | 1-2 days (longer in patients with renal impairment) |
| Rivaroxaban | Factor Xa inhibitor | 5-9 hours | AF, VTE, VTE after surgery | 1-2 days (longer in patients with renal impairment) |
| Edoxaban | Factor Xa inhibitor | 10-14 hours | AF, VTE, VTE after surgery | 1-2 days (longer in patients with renal impairment) |
| Apixaban | Factor Xa inhibitor | 12 hours | AF, VTE, VTE after surgery | 1-2 days (longer in patients with renal impairment) |
| ACS, acute coronary syndrome; AF, atrial fibrillation; AIS, acute ischemic stroke; ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; COX, cyclooxygenase; DAPT, dual antiplatelet therapy; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention (stent); PE, pulmonary embolism; VTE, venous thromboembolism. | ||||
Other antiplatelet agents — clopidogrel, ticagrelor, and prasugrel — inhibit ADP-mediated platelet activation by blocking the P2Y12 receptor. These agents work synergistically with aspirin and are commonly used in DAPT in conjunction with aspirin. Ticagrelor is a direct-acting reversible blocker of P2Y12 receptor. It has the fastest onset of action (within 30 minutes) of all antiplatelet agents and achieves more potent inhibition of platelet activation compared to clopidogrel.7
Clopidogrel, the most commonly prescribed P2Y12 inhibitor, requires 2-step metabolism in the liver to produce an active metabolite that rapidly and irreversibly binds and inhibits the P2Y12 receptor on the platelet surface for the remainder of their lifespan (7 to 10 days). The onset of action for clopidogrel is 2 to 6 hours, the slowest of all antiplatelet agents. Some patients who are unable to metabolize clopidogrel to its active metabolite may not be responsive to it.
Prasugrel is a thienopyridine, like clopidogrel, which also needs to be converted to an active metabolite by the cytochrome P450–dependent pathway. It has a faster onset of action (30 to 60 minutes) compared to clopidogrel and is the most potent of all P2Y12 inhibitors. It also has the highest risk of major bleeding among all antiplatelet agents, particularly in patients older than 75 years, and those weighing less than 60 kg. It is indicated only for acute coronary syndromes (unstable angina or myocardial infarction) managed with percutaneous coronary intervention (stents), and it carries an FDA black box warning to discontinue at least 7 days prior to any surgery. It is contraindicated in patients with a history of stroke or transient ischemic attack due to an increased risk of intracranial bleed in this subgroup.8
The time for platelet function restoration after interruption of P2Y12 blocking agents is 5 days for clopidogrel, 3 days for ticagrelor, and 7 days for prasugrel.9 The order of potency of AP agents from greatest to least is prasugrel, ticagrelor, clopidogrel, and aspirin. Both prasugrel and ticagrelor are superior to clopidogrel in cardiovascular outcomes following percutaneous coronary intervention, but they carry an increased risk for bleeding, particularly with prasugrel.10
Antiplatelet agents are effective in preventing arterial thrombosis and are commonly employed for coronary artery disease, peripheral arterial disease, and secondary prevention following myocardial infarction and ischemic stroke. Dual antiplatelet therapy, consisting of aspirin and a P2Y12 inhibitor, is recommended by the American College of Cardiology for at least 1 year following an acute coronary syndrome (ACS), defined as myocardial infarction or unstable angina regardless of intervention (medical therapy, percutaneous coronary intervention [PCI], or coronary artery bypass surgery), followed by 81 mg/day aspirin indefinitely.10,11 Current guidelines favor ticagrelor and prasugrel over clopidogrel.10 Stent thrombosis, the most dreaded complication of DAPT interruption, which can result in acute myocardial infarction and death, is less common with modern second-generation and third-generation drug-eluting stents (DES) compared to bare metal stents (BMS) and first-generation DES. The risk of stent thrombosis is highest in the first month after BMS and first 3 months after DES. It decreases with the passage of time, and American College of Cardiology guidelines permit temporary perioperative interruption of DAPT after 6 months following PCI in selected patients at particularly high risk for bleeding, preferably with intraoperative continuation of aspirin.10,12 The PARIS registry showed that short-term perioperative interruption of DAPT is not particularly dangerous if it is done after 6 months from PCI and if only 1 agent is interrupted.13 Recent data from the TICO and TWILIGHT trials have demonstrated that with newer generation DES, a short DAPT duration (<3 months) or even monotherapy with ticagrelor is safe and reduces major bleeding risk in patients at high risk of bleeding after PCI.14,15 However, the ischemic and bleeding risk should be balanced in each patient, and this decision should not be made without consulting the patient’s cardiologist, especially in patients with PCI less than 1 year earlier. Dual therapy with aspirin plus clopidogrel or ticagrelor for 21 days followed by aspirin monotherapy is the most recommended AP therapy for secondary prevention following acute ischemic stroke, transient ischemic attack, or retinal artery occlusion.16
The role of aspirin in primary prevention of atherosclerotic cardiovascular disease (ASCVD) has become controversial in recent years. Approximately 25% of US adults older than 40 years without a history of heart disease or stroke take aspirin. According to a recent review, this may lead to slight cardiovascular benefit (absolute risk reduction 0.41%) but an equally increased risk of major bleeding (absolute risk increase 0.47%).17 The American College of Cardiology no longer recommends aspirin use for primary prevention of ASCVD in patients 70 years or older but allows it to be considered in selected patients between ages 40 and 70 who have high-risk characteristics.18 For patients taking aspirin for primary prevention of ASCVD, it is generally safe and reasonable to interrupt aspirin perioperatively.
ANTICOAGULANTS
Anticoagulant agents prevent formation and enlargement of blood clots. Oral anticoagulant agents belong to 1 of 2 classes: vitamin K antagonists (VKAs), such as warfarin, and direct-acting oral anticoagulants (DOACs), also known as novel oral anticoagulants, which include the thrombin (factor IIa) inhibitor dabigatran etexilate (Pradaxa; Boehringer Ingelheim) and factor Xa inhibitors rivaroxaban (Xarelto; Janssen), apixaban, and edoxaban (Savaya; Daiichi Sankyo). Anticoagulants are more effective on the venous side and are commonly used for stroke prophylaxis in atrial fibrillation, deep vein thrombosis, pulmonary embolism, mechanical cardiac valve, cardiomyopathies, and hypercoagulable states such as lupus anticoagulant and factor V deficiency or malignancy.
Warfarin is a VKA that inhibits formation of coagulation factors II, VII, IX and X. It has a variable half-life of 36 hours to 42 hours requiring periodic monitoring of international normalized ratio (INR). For most indications, target INR on warfarin is between 2.0 to 3.0, except for mechanical mitral or combined mitral and aortic valves where target INR should be between 2.5 and 3.5.19 It may take about 3 days after warfarin interruption for the INR to fall below 2 (subtherapeutic). After resumption of warfarin, it may take about 3 days for INR to reach therapeutic levels.20 If needed, oral or parenteral vitamin K can reverse anticoagulation from warfarin in 12 to 24 hours.19
Perioperative bridging with low molecular weight heparin in patients with atrial fibrillation during warfarin interruption does not reduce the incidence of systemic thromboembolism and may even be harmful by causing a 4-fold increase in bleeding.21 Bridging is therefore not indicated for most patients receiving warfarin, except for those with a mechanical heart valve, recent thromboembolic event, and systemic coagulopathies.6,21
Direct-acting oral anticoagulants have rapid onset of action (1 to 2 hours), with peak serum concentration in 3 to 4 hours and short half-lives ranging from 7 to 17 hours.22 They have a large therapeutic window that allows correct plasma concentration in patients over a wide weight range (40 kg to 120 kg) with the same standard dose without any need for monitoring. Dabigatran etexilate is converted to active metabolite dabigatran in the liver and inhibits thrombin (factor IIa), preventing it from converting fibrinogen to fibrin during coagulation cascade.
Rivaroxaban, apixaban, and edoxaban are direct inhibitors of factor Xa which converts prothrombin (factor II) to thrombin (factor IIa). Dabigatran and apixaban are administered twice daily, whereas rivaroxaban and edoxaban are dosed once daily. Apixaban has become popular because it has similar efficacy to other direct-acting oral anticoagulants, but less risk of bleeding, particularly gastrointestinal.23 Novel oral anticoagulants are currently preferred agents over warfarin for stroke prophylaxis in nonvalvular atrial fibrillation and most other indications except mechanical cardiac valve.24 A recent meta-analysis confirmed superiority of direct-acting oral anticoagulants over warfarin for stroke prophylaxis in atrial fibrillation patients and favored apixaban over other direct-acting oral anticoagulants.25 All direct-acting oral anticoagulants are cleared primarily by the kidneys and are contraindicated in patients with severe kidney disease (creatinine clearance below 30 mL/min), in which case warfarin is preferred.
Because DOACs have short half-lives, interruption for 1 to 2 days prior to a surgical procedure is sufficient without the need for bridging with low molecular weight heparin. The American College of Cardiology recommends holding direct-acting oral anticoagulants for 2 half-lives prior to low bleeding risk procedures and 5 half-lives prior to intermediate/high/uncertain bleeding risk procedures.26 Antiplatelet agents and anticoagulants need not be interrupted prior to procedures with no clinically important bleeding risk (eg, intravitreal injections, clear cornea cataract surgery, periocular injections) and should be resumed after adequate hemostasis has been ensured, preferably within 24 to 48 hours after surgery.27
Two reversal agents for DOACs have recently been approved by the FDA: idarucizumab (Praxbind; Boehringer Ingelheim) for dabigatran and andexanet alfa (Adnexxa; Alexion) for apixaban and rivoraxaban.28 However, these reversal agents are expensive and not readily available. Patients on both antiplatelet and anticoagulant agents, for example DAPT following stents in the setting of atrial fibrillation (triple therapy), may be at a particularly high bleeding risk.
SYSTEMIC CONDITIONS THAT INCREASE THE RISK OF BLEEDING
A simple and readily available score, HAS-BLED, originally developed to assess the risk of major bleeding in anticoagulated atrial fibrillation patients, can be used quickly to screen for major systemic conditions that increase the risk of perioperative bleeding (https://www.mdcalc.com/has-bled-score-major-bleeding-risk ).29 The components of the HAS-BLED score are hypertension or systolic blood pressure >160 mmHg (H); abnormal liver/renal function (A); stroke history (S); bleeding history or predisposition (B); labile INR (L); elderly (E); drugs, such as antiplatelets or anticoagulants, and alcohol (D).
Of these, hypertension can and should be managed appropriately prior to proceeding with vitreoretinal surgery. Perioperative hypertension is a risk factor for surgical site bleeding and adverse cardiovascular events. The 2017 guidelines from the American College of Cardiology suggest deferring an elective surgery for systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg.30 Some anesthesia departments have policies to hold diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers on the day of eye surgery performed under local anesthesia; these guidelines unnecessarily put the patients at risk of unsafe perioperative hypertension. A surgeon should develop the habit of measuring the patient’s blood pressure prior to proceeding with the block.
DEFINITION OF MAJOR BLEEDING
An intraocular bleed that compromises vision is considered a major bleed (type 3c, in the same category as intracranial bleed) according to Bleeding Academic Research Consortium (BARC) criteria, and in several other clinical trials.31 In the medical literature, surgeries with ≥4% risk of major bleed are considered high bleeding risk procedures.31 A major surgery lasting more than 45 minutes is also classified as high bleeding risk procedure.32 Anesthesia literature considers “posterior chamber eye surgery” as high surgical hemorrhagic risk.33 With those perspectives, most retinal procedures with the exception of simple macular hole, macular pucker, floater removal, or asteroid hyalosis will qualify as high bleeding risk procedures and justify appropriate perioperative adjustment of AP/AC agents.
Certain retinal surgeries, such as diabetic vitrectomy with extensive fibrovascular membranes, sutured IOL, scleral buckle with external drainage of subretinal fluid, chorioretinal biopsy, submacular surgery, surgery for hemorrhagic complications of peripheral exudative hemorrhagic chorioretinopathy, and proliferative vitreoretinopathy surgery involving large retinectomies may be at particularly high risk for perioperative bleeding (Figures 1 and 2). Other ocular factors that increase the risk of perioperative bleeding including suprachoroidal hemorrhage include myopia, glaucoma, inflammation, and hypotony.34,35
REVIEW OF RETINAL LITERATURE
Anticoagulant and AP agents may increase the risk of submacular hemorrhage in neovascular age-related macular degeneration and of vitreous hemorrhage after acute posterior vitreous detachment.36-38 Dual antiplatelet therapy and AC may increase the risk of orbital hemorrhage after retrobulbar or peribulbar anesthesia.39 Cases of intractable bleeding during diabetic vitrectomy and complex retinal detachment surgery requiring retinectomy in patients receiving DAPT have been reported.40,41 Direct-acting oral anticoagulants have been associated with spontaneous vitreous hemorrhage.42,43
Antiplatelet agents should not be interrupted for intravitreal injections.44 Studies on perioperative antithrombotic therapy in vitreoretinal surgery are conflicting and inconclusive due to short sample size and a myriad of unmeasured/uncontrolled confounding variables and are summarized in Table 2. Antithrombotic agents were likely held perioperatively in significant patients in most studies,45,46 and this crucial information was not available in most (Table 2). Vitreoretinal surgery was performed for a variety of indications in most studies. None performed a thorough analysis of systemic factors that increase bleeding risk. There were very few patients on DAPT, more potent AP agents, or DOACs. Information regarding INR on the day of surgery is not available in most studies. Some studies have shown increased risk of intraoperative hemorrhage in patients on aspirin or warfarin,47-49 while others have not.
| STUDY (YEAR) | NATURE OF STUDY | N, SURGICAL INDICATION | RESULTS | COMMENTS |
| Lauermann et al (2021)51 | P, NR | 374 (total), scleral buckle and PPV for a variety of indications. 134 (36%) on AP/AC (86 ASA). 47 discontinued AP/AC, 7 bridged. | 15/374 (4%) overall risk of severe intraoperative bleeding (6/134 4.5% AP/AC). Increased risk with DM, carotid artery stenosis, diabetic retinopathy, young age, transscleral drainage, not AC/AP. | 82 patients continued AP/AC perioperatively. Of these, 3 (1 ASA, 1 clopidogrel, 1 phenprocoumon) had severe bleeding. Very few on newer AP. Most patients on DOAC discontinued them. |
| Louison et al (2020)54 | P, NR, MC | 748 PPV for macular surgery. 202 (27%) on AT (146 AP, 47 AC, 6 AP+AC). | 92 (12.3%) hemorrhagic complications. No difference between AT and non AT. | 10 on DAPT, 10 on clopidogrel, and 24 on DOAC. Discontinuation defined as >3 days ASA rivoraxaban, apixaban, >4 days dabigatran >5 days clopidogrel, warfarin. |
| Bemme (2020)45 | CC | 893 patients with RRD. 192(21.5%) on AT, 701 controls. RRD repaired by a variety of techniques. | “No increased rate of perioperative hemorrhage.” | 1. External drainage of SRF risk factor for bleeding 2. All patients in phenprocoumon (vitamin K antagonist) had subtherapeutic INR 3. Aspirin group defined as aspirin <10 days prior to surgery. 4. Very few patients on clopidogrel, none on newer P2Y12 inhibitors (probably none on DAPT). |
| Meillon et al (2018)55 | P, NC, MC | Total 804. 148 (18.4%) AP, 63 (7.8%) AC, | 53/804 (6.6%) hemorrhagic complications. AP/AC not associated with bleeding; only endodiathermy use was. | Discontinuation definition >3 days for AP, >5 days for warfarin and DOAC. SCH, subretinal and choroidal bleed more common in AP (6.1% v 1.1%). Only 12 on DAPT. Median INR measured day before surgery was 1.95 (subtherapeutic). |
| Grand (2016)52 | R, NC | 33 eyes on NOAC or prasurgel, variety of indications. | No case of bleeding. 4 eyes (12%) developed postoperative VH. | Not known if AP or DOACs were temporarily held. 8/21 eyes been on prasugrel >1 year after PCI (unusual). DOACs were for AF and could have been safely interrupted. Small sample size. |
| Brillat (2015)46 | P, CC | 835 eyes with RRD. 74 eyes with bleed. 248 randomly selected controls. | Aspirin not a risk factor for bleeding; cryotherapy, external drainage, and PPV were. | Only 28 patients (8.6%) were in aspirin group. Even in that group, 19 interrupted aspirin >2 days prior to surgery. Did not evaluate other AP/AC agents. |
| Chandra (2014)53 | R, CC | 5,459 patients undergoing PPV over 10 years. | 56 eyes (1.03%) developed SCH (cases), rest control. Age, male sex, RRD, dropped cataract, scleral exoplant, RRD, aspirin/warfarin were risk factors. | Highest risk of SCH in dropped lens cases. Not known if AP/AC agents were temporarily held. |
| Ryan (2013)56 | P | 85 patients, on ASA, clopidogrel, or warfarin undergoing PPV for a variety of indications. | 23% intraoperative bleed, 22% postoperative bleed. | No case of choroidal hemorrhage or uncontrolled hemorrhage. PDR risk factor for bleeding. 72% procedures on ASA alone, 7.5% on clopidogrel alone, 10% warfarin. |
| Khuthaila (2013)57 | R, NC | 173 eyes undergoing PPV for nonclearing diabetic VH. | 56 developed postop VH. Young age, phakia, incomplete PRP prior to PPV were risk factors. | No association with AC/AP use and postop VH. Not known if AC/AP were interrupted prior to surgery. |
| Passemard (2012)47 | R, C | 206 eyes, PPV for various indications. 30% were on AT, 12 (5.8%) on AC, 44 (21.4%) on AP, 6 on AP+AC, 144 control. | 20% developed severe bleeding in AP group. | Very small numbers. No patients on newer AP agents ticagrelor or prasugrel. |
| Malik (2012)58 | R, NC | 52 patients on a variety of AC/AP regimens, 32 ASA+CPG, 18 warfarin, 3 ASA+warfarin, 2 ASA+dipyridamole, 1 CPG+warfarin. 23-gauge PPV for a variety of indications. | No intraoperative bleeding, 13% postoperative vitreous hemorrhage. | Subconjunctival anesthesia. No discontinuation of AT. |
| Fabinyi (2011)48 | R, C | 139 patients (155 eyes) undergoing diabetic PPV. 68 (44%) on AT. 39 interrupted AP/AC prior to surgery. | Perioperative continuation of AP and AC increased the risk of persistent VH. | Consider AT interruption in diabetic vitrectomy. |
| Chandra (2011)59 | R, CC | 60 patients on warfarin, 60 matched controls. | No increase in complications in patients on warfarin. | 26% in warfarin group had INR <2. |
| Brown (2011)60 | R, C | 97 eyes undergoing diabetic PPV; 27 remained on AC/AP. | No increase in risk of intraoperative or postoperative hemorrhage in those on AC/AP. | Very small numbers. 16/27 on ASA alone. 10 on DAPT (ASA+clopidogrel). |
| Mason (2011)2 | R, NC | 289 consecutive patients. 61 patients on warfarin, 118 on CPG. 25-gauge PPV for various indications. | No choroidal or retrobulbar hemorrhage. About 4% transient VH in CPG group. | 43/61 patients in warfarin group had INR <2. INR obtained <5 days prior to surgery (not on day of surgery). |
| Oh (2011)1 | R, CC | 422 eyes undergoing PPV. | Higher bleeding risk in AP and AC group. | Less bleeding risk if ASA interrupted for 3 days. |
| Fu (2007)61 | R, NC | 25 patients on warfarin undergoing VR surgery. | 1 subretinal hemorrhage from external drainage (INR 2.7). | 11/25 had subtherapeutic INR (<2). |
| Dayani (2006)62 | R, C | 57 patients on warfarin undergoing VR surgery. | No increase in risk of perioperative bleed. | 35/57 patients had subtherapeutic INR (<2). |
| Narendran (2003)49 | R, C | 541 consecutive eyes undergoing PPV. 60 on aspirin, 7 on warfarin. | Warfarin risk factor for vitreous and choroidal bleed. Aspirin was not. | Very small numbers. Older study predates more potent AP agents. |
| AF, atrial fibrillation; AP/AC: antiplatelet/anticoagulant; ASA, acetylsalicylic acid or aspirin; AT, antithrombotics; C, comparative; CC, case control; CPG, clopidogrel; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; DOAC, direct oral anticoagulants; INR, international normalized ratio; MC, multicenter; NC, noncomparative; P, prospective; PPV, pars plana vitrectomy; PRP, panretinal photocoagulation; R, retrospective; RRD, rhegmatogenous retinal detachment; SCH, suprachoroidal hemorrhage; VH, vitreous hemorrhage; VR, vitreoretinal. | ||||
Bemme et al found no association between AC/AP use in retinal detachment surgery.45 However, all patients in the vitamin K antagonist group had subtherapeutic INR and the aspirin group (incorrectly) included anyone who took aspirin ≤10 days prior to surgery, making their conclusions invalid.50 Brillat et al found that aspirin use, defined as aspirin interruption equal to or less than 5 days prior to surgery, was not associated with an increased risk of bleeding. They found cryotherapy, transscleral drainage of subretinal fluid, and pars plana vitrectomy to be risk factors for perioperative bleeding.46 Other authors have also used arbitrary cutoffs to define discontinuation (Table 2). Severe bleeding events have been reported after seemingly low-risk procedures. For example, Lauermann et al reported severe bleeding after removal of silicone oil (3/55 eyes), vitreous floater removal (1/3 eyes), uncomplicated retinal detachment (3/59 eyes), and simple vitreous hemorrhage (1/24 eyes).51
Grand and Walia found no case of intraocular or periocular hemorrhage during pars plana vitrectomy performed for a variety of indications in 33 eyes on DOAC or prasugrel therapy.52 Chandra et al found warfarin and aspirin use to be associated with an increased risk for intraoperative suprachoroidal hemorrhage during pars plana vitrectomy.53 Numerous other risk factors for bleeding identified in these studies include, age, male sex, rhegmatogenous retinal detachment surgery, buckle placement, external drainage of subretinal fluid, cryotherapy, proliferative diabetic retinopathy especially with incomplete preoperative panretinal photocoagulation, and vitrectomy for retained cataract fragments (Table 2). Patients undergoing diabetic vitrectomy appear to have high incidence of intraoperative bleeding and postoperative vitreous hemorrhage. Intravitreal anti-VEGF injection 3 days to 8 days prior to vitrectomy may be considered in these patients.
CONCLUSION
Modern vitrectomy surgery enables more control of intraoperative bleeding compared to many other types of surgeries. Still, given the seriousness and potentially blinding consequences of intraocular bleeding and recognition of intraocular bleed as “severe” bleeding in the medical literature, it is reasonable to attempt to optimize antithrombotic agents and systemic status prior to vitreoretinal surgery, if possible. A clear communication with the patient’s medical providers outlining the bleeding risk of proposed surgery and expected duration of antithrombotic interruption is desirable to optimize the patient’s preoperative status and improve outcomes. The following general suggestions are offered to help accomplish these goals.
- Categorize the patient’s perioperative bleeding risk into low, intermediate, or high based on the complexity of surgical procedure, systemic factors (HAS-BLED), and the patient’s ocular comorbidities, such as myopia, glaucoma, inflammation, and hypotony.
- Categorize thrombotic risk into low, intermediate, or high based on type of stent and time since PCI.12
- In general, elective surgery should be avoided if possible in the first 6 months to 12 months after acute coronary syndrome, as recommended by the American College of Cardiology.
- Consider a detailed discussion with the patient and appropriate communication with other health care providers regarding options in elective cases, including the choice of anesthesia (local in most cases, particularly those with significant systemic comorbid conditions), duration of surgery, and expected duration of interruption of antithrombotic agents. Because these are complex issues, it is advisable to carefully document your discussion, particularly noting the increased bleeding risk due to antithrombotic agents. A special consent form, such as one designed by the Ophthalmic Mutual Insurance Company (https://www.omic.com/hemorrhage-associated-with-ophthalmic-procedures/ ) may be considered to streamline this process.
- Do not interrupt AC/AP agents any longer than necessary.
- According to the medical literature, anticoagulants — both VKAs and DOACs — may be held in most cases without bridging (exceptions include mechanical heart valve, recent thromboembolic episode, and some systemic coagulopathies).
- The generally safe and adequate period of preoperative interruption, approximately equal to 3 elimination half-lives of active compound, is 2 days for DOAC; 3 days for aspirin, ticagrelor, and warfarin; 5 days for clopidogrel; and 7 days for prasugrel (Table 1). If a patient is on DAPT, it is preferable not to stop both agents within the first year. Instead, consider continuing aspirin perioperatively, including on the day of surgery, in consultation with the patient’s cardiologist.
- Consider making adjustments in surgical technique where AC and AP agents can’t be interrupted, or for surgeries performed as an emergency for macula-on retinal detachment or endophthalmitis (sub-Tenon as opposed to retrobulbar block, minimize cryotherapy, avoid hypotony, avoid external drainage, if possible, electively suture sclerotomies especially in myopic eyes, 400 μg intravitreal dexamethasone in inflamed eyes or those receiving extensive panretinal photocoagulation, ensure hemostasis) (Video 1).
Video 1. A simple technique of posterior sub-Tenon anesthetic injection. This technique eliminates potential complications from peribulbar or retrobulbar injections. - Consider resumption of antithrombotic agents soon after surgery, generally on the first or second postoperative day, unless the patient has hypotony or choroidal detachments where longer interruption in consultation with medical providers may be considered.
- Do not hold antihypertensive agents except for diuretics on the day of surgery. Be aware of the patient’s blood pressure, and have the anesthesia provider control it if systolic blood pressure is >160 mmHg. RP
-
References
- Oh J, Smiddy WE, Kim SS. Antiplatelet and anticoagulation therapy in vitreoretinal surgery. Am J Ophthalmol. 2011;151(6):934-939.e3. doi:10.1016/j.ajo.2010.09.035
- Mason JO, Gupta SR, Compton CJ, et al. Comparison of hemorrhagic complications of warfarin and clopidogrel bisulfate in 25-gauge vitrectomy versus a control group. Ophthalmology. 2011;118(3):543-547. doi:10.1016/j.ophtha.2010.07.005
- Bucsi R. Monocular patient loses vision after vitrectomy. Ophthalmic Mutual Insurance Company Digest. 2014;24(1). Accessed May 11, 2022. https://www.omic.com/monocular-patient-loses-vision-after-vitrectomy/
- Jourdi G, Godier A, Lordkipanidzé M, Marquis-Gravel G, Gaussem P. Antiplatelet therapy for atherothrombotic disease in 2022—from population to patient-centered approaches. Front Cardiovasc Med. 2022;9:805525. doi:10.3389/fcvm.2022.805525
- Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW. Reversal of the anti-platelet effects of aspirin and clopidogrel. J Thromb Haemost. 2012;10(4):521-528. doi:10.1111/j.1538-7836.2012.04641.x
- Smilowitz NR, Berger JS. Perioperative cardiovascular risk assessment and management for noncardiac surgery: a review. JAMA. 2020;324(3):279-290. doi:10.1001/jama.2020.7840
- Dobesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34(10):1077-1090. doi:10.1002/phar.1477
- Norgard NB, Abu-Fadel M. Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc Health Risk Manag. 2009;5:873-882. doi:10.2147/vhrm.s5699
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The task force for dual antiplatelet therapy in coronary artery disease of the European society of cardiology (ESC) and of the European association for cardio-thoracic surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):123. doi:10.1161/CIR.0000000000000404
- Kamran H, Jneid H, Kayani WT, et al. Oral antiplatelet therapy after acute coronary syndrome: a review. JAMA. 2021;325(15):1545-1555. doi:10.1001/jama.2021.0716
- Banerjee S, Angiolillo DJ, Boden WE, et al. Use of antiplatelet therapy/DAPT for Post-PCI patients undergoing noncardiac surgery. J Am Coll Cardiol. 2017;69(14):1861-1870. doi:10.1016/j.jacc.2017.02.012
- Schoos M, Chandrasekhar J, Baber U, et al. Causes, timing, and impact of dual antiplatelet therapy interruption for surgery (from the patterns of non-adherence to anti-platelet regimens in stented patients registry). Am J Cardiol. 2017;120(6):904-910. doi:10.1016/j.amjcard.2017.06.016
- Dangas G, Baber U, Sharma S, et al. Ticagrelor with or without aspirin after Complex PCI. J Am Coll Cardiol. 2020;75(19):2414-2424. doi:10.1016/j.jacc.2020.03.011
- Kim B, Hong S, Cho Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323(23):2407-2416. doi:10.1001/jama.2020.7580
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
- Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198(4):198-199. doi:10.5694/mja12.10614
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):160S-198S. doi:10.1378/chest.08-0670
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833. doi:10.1056/NEJMoa1501035
- Sunkara T, Ofori E, Zarubin V, Caughey ME, Gaduputi V, Reddy M. Perioperative management of direct oral anticoagulants (DOACs): a systemic review. Health Serv Insights. 2016;9(Suppl 1):25-36. doi:10.4137/HSI.S40701
- Noseworthy PA, Yao X, Shah ND, Gersh BJ. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease. Int J Cardiol. 2016;209:181-183. doi:10.1016/j.ijcard.2016.02.005
- Beaser AD, Cifu AS. Management of patients with atrial fibrillation. JAMA. 2019;321(11):1100-1101. doi:10.1001/jama.2019.1264
- López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. doi:10.1136/bmj.j5058
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76(5):594-622. doi:10.1016/j.jacc.2020.04.053
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469-1478. doi:10.1001/jamainternmed.2019.2431
- Mujer MTP, Rai MP, Atti V, et al. An update on the reversal of non-vitamin K antagonist oral anticoagulants. Adv Hematol. 2020;2020:7636104. doi:10.1155/2020/7636104
- Santise G, Nardella S, Migliano F, Testa A, Maselli D. The HAS-BLED score is associated with major bleeding in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(6):1601-1606. doi:10.1053/j.jvca.2019.01.021
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736-2747. doi:10.1161/CIRCULATIONAHA.110.009449
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120(15):2954-2962. doi:10.1182/blood-2012-06-415943
- Oprea AD, Popescu WM. Perioperative management of antiplatelet therapy. Br J Anaesth. 2013;111 Suppl 1:3. doi:10.1093/bja/aet402
- Lakhanpal V, Schocket SS, Elman MJ, Dogra MR. Intraoperative massive suprachoroidal hemorrhage during pars plana vitrectomy. Ophthalmology. 1990;97(9):1114-1119. doi:10.1016/s0161-6420(90)32448-x
- Tabandeh H, Sullivan PM, Smahliuk P, Flynn HW, Schiffman J. Suprachoroidal hemorrhage during pars plana vitrectomy. risk factors and outcomes. Ophthalmology. 1999;106(2):236-242. doi:10.1016/S0161-6420(99)90062-3
- Kiernan DF, Hariprasad SM, Rusu IM, Mehta SV, Mieler WF, Jager RD. Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010;30(10):1573-1578. doi:10.1097/IAE.0b013e3181e2266d
- Kuhli-Hattenbach C, Fischer IB, Schalnus R, Hattenbach L. Subretinal hemorrhages associated with age-related macular degeneration in patients receiving anticoagulation or antiplatelet therapy. Am J Ophthalmol. 2010;149(2):316-321.e1. doi:10.1016/j.ajo.2009.08.033
- Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2013;33(3):621-626. doi:10.1097/IAE.0b013e3182671006
- Huebert I, Heinicke N, Kook D, et al. Dual platelet inhibition in cases of severe retrobulbar hemorrhage following retrobulbar and peribulbar anesthesia. J Cataract Refract Surg. 2015;41(10):2092-2101. doi:10.1016/j.jcrs.2015.10.051
- Herbert EN, Mokete B, Williamson TH, Laidlaw DaH. Haemorrhagic vitreoretinal complications associated with combined antiplatelet agents. Br J Ophthalmol. 2006;90(9):1209-1210. doi:10.1136/bjo.2006.095307
- Zarei M. Dual antiplatelet therapy may lead to hemorrhagic complications in vitrectomy of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):425-426. doi:10.1007/s00417-018-4151-1
- Shieh W, Sridhar J, Hong BK, et al. Ophthalmic complications associated with direct oral anticoagulant medications. Semin Ophthalmol. 2017;32(5):614-619. doi:10.3109/08820538.2016.1139738
- Jun JH, Hwang JC. Association of rivaroxaban anticoagulation and spontaneous vitreous hemorrhage. JAMA Ophthalmol. 2015;133(10):1184-1186. doi:10.1001/jamaophthalmol.2015.2069
- Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34 Suppl 12:S1-S18. doi:10.1097/IAE.0000000000000399
- Bemme S, Lauermann P, Striebe NA, et al. Risk of perioperative bleeding complications in rhegmatogenous retinal detachment surgery: a retrospective single-center study. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):961-969. doi:10.1007/s00417-019-04554-1
- Brillat E, Rouberol F, Palombi K, et al. A case-control study to assess aspirin as a risk factor of bleeding in rhegmatogenous retinal detachment surgery. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1899-1905. doi:10.1007/s00417-014-2900-3
- Passemard M, Koehrer P, Juniot A, Bron AM, Creuzot-Garcher C. Maintenance of anticoagulant and antiplatelet agents for patients undergoing peribulbar anesthesia and vitreoretinal surgery. Retina. 2012;32(9):1868-1873. doi:10.1097/IAE.0b013e31825097ae
- Fabinyi DCA, O'Neill EC, Connell PP, Clark JB. Vitreous cavity haemorrhage post-vitrectomy for diabetic eye disease: the effect of perioperative anticoagulation and antiplatelet agents. Clin Exp Ophthalmol. 2011;39(9):878-884. doi:10.1111/j.1442-9071.2011.02575.x
- Narendran N, Williamson TH. The effects of aspirin and warfarin therapy on haemorrhage in vitreoretinal surgery. Acta Ophthalmol Scand. 2003;81(1):38-40. doi:10.1034/j.1600-0420.2003.00020.x
- Kishore K, Mungee S. Comment on “risk of perioperative bleeding complications in rhegmatogenous retinal detachment surgery: a retrospective single-center study”: Bemme S et al. Graefes Arch Clin Exp Ophthalmol. 2020; 258(5). doi:10.1007/s00417-019-04554-1. Graefes Arch Clin Exp Ophthalmol. 2020;258(9):2071-2072. doi:10.1007/s00417-020-04723-7
- Lauermann P, Klingelhöfer A, Mielke D, et al. Risk factors for severe bleeding complications in vitreoretinal surgery and the role of antiplatelet or anticoagulant agents. Ophthalmol Retina. 2021;5(8):e23-e29. doi:10.1016/j.oret.2021.04.013
- Grand MG, Walia HS. Hemorrhagic risk of vitreoretinal surgery in patients maintained on novel oral anticoagulant therapy. Retina. 2016;36(2):299-304. doi:10.1097/IAE.0000000000000783
- Chandra A, Xing W, Kadhim MR, Williamson TH. Suprachoroidal hemorrhage in pars plana vitrectomy: risk factors and outcomes over 10 years. Ophthalmology. 2014;121(1):311-317. doi:10.1016/j.ophtha.2013.06.021
- Louison S, Gabrielle P, Soudry A, et al. Perioperative risk of bleeding with antithrombotic agents in macular surgery: a national, prospective, multicentre study. Acta Ophthalmol. 2020;98(8):e991-e997. doi:10.1111/aos.14434
- Meillon C, Gabrielle PH, Luu M, Aho-Glele LS, Bron AM, Creuzot-Garcher C. Antiplatelet and anticoagulant agents in vitreoretinal surgery: a prospective multicenter study involving 804 patients. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):461-467. doi:10.1007/s00417-017-3897-1
- Ryan A, Saad T, Kirwan C, Keegan DJ, Acheson RW. Maintenance of perioperative antiplatelet and anticoagulant therapy for vitreoretinal surgery. Clin Exp Ophthalmol. 2013;41(4):387-395. doi:10.1111/ceo.12017
- Khuthaila MK, Hsu J, Chiang A, et al. Postoperative vitreous hemorrhage after diabetic 23-gauge pars plana vitrectomy. Am J Ophthalmol. 2013;155(4):757-763. doi:10.1016/j.ajo.2012.11.004
- Malik AI, Foster RE, Correa ZM, Petersen MR, Miller DM, Riemann CD. Anatomical and visual results of transconjunctival sutureless vitrectomy using subconjunctival anesthesia performed on select patients taking anticoagulant and antiplatelet agents. Retina. 2012;32(5):905-911. doi:10.1097/IAE.0b013e31822f55c4
- Chandra A, Jazayeri F, Williamson TH. Warfarin in vitreoretinal surgery: a case controlled series. Br J Ophthalmol. 2011;95(7):976-978. doi:10.1136/bjo.2010.187526
- Brown JS, Mahmoud TH. Anticoagulation and clinically significant postoperative vitreous hemorrhage in diabetic vitrectomy. Retina. 2011;31(10):1983-1987. doi:10.1097/IAE.0b013e31821800cd
- Fu AD, McDonald HR, Williams DF, et al. Anticoagulation with warfarin in vitreoretinal surgery. Retina. 2007;27(3):290-295. doi:10.1097/01.iae.0000243033.39301.10
- Dayani PN, Grand MG. Maintenance of warfarin anticoagulation for patients undergoing vitreoretinal surgery. Trans Am Ophthalmol Soc. 2006;104:149-160.
Video 1. A simple technique of posterior sub-Tenon anesthetic injection. This technique eliminates potential complications from peribulbar or retrobulbar injections.
- Consider resumption of antithrombotic agents soon after surgery, generally on the first or second postoperative day, unless the patient has hypotony or choroidal detachments where longer interruption in consultation with medical providers may be considered.
- Do not hold antihypertensive agents except for diuretics on the day of surgery. Be aware of the patient’s blood pressure, and have the anesthesia provider control it if systolic blood pressure is >160 mmHg. RP
References
- Oh J, Smiddy WE, Kim SS. Antiplatelet and anticoagulation therapy in vitreoretinal surgery. Am J Ophthalmol. 2011;151(6):934-939.e3. doi:10.1016/j.ajo.2010.09.035
- Mason JO, Gupta SR, Compton CJ, et al. Comparison of hemorrhagic complications of warfarin and clopidogrel bisulfate in 25-gauge vitrectomy versus a control group. Ophthalmology. 2011;118(3):543-547. doi:10.1016/j.ophtha.2010.07.005
- Bucsi R. Monocular patient loses vision after vitrectomy. Ophthalmic Mutual Insurance Company Digest. 2014;24(1). Accessed May 11, 2022. https://www.omic.com/monocular-patient-loses-vision-after-vitrectomy/
- Jourdi G, Godier A, Lordkipanidzé M, Marquis-Gravel G, Gaussem P. Antiplatelet therapy for atherothrombotic disease in 2022—from population to patient-centered approaches. Front Cardiovasc Med. 2022;9:805525. doi:10.3389/fcvm.2022.805525
- Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW. Reversal of the anti-platelet effects of aspirin and clopidogrel. J Thromb Haemost. 2012;10(4):521-528. doi:10.1111/j.1538-7836.2012.04641.x
- Smilowitz NR, Berger JS. Perioperative cardiovascular risk assessment and management for noncardiac surgery: a review. JAMA. 2020;324(3):279-290. doi:10.1001/jama.2020.7840
- Dobesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34(10):1077-1090. doi:10.1002/phar.1477
- Norgard NB, Abu-Fadel M. Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc Health Risk Manag. 2009;5:873-882. doi:10.2147/vhrm.s5699
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The task force for dual antiplatelet therapy in coronary artery disease of the European society of cardiology (ESC) and of the European association for cardio-thoracic surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):123. doi:10.1161/CIR.0000000000000404
- Kamran H, Jneid H, Kayani WT, et al. Oral antiplatelet therapy after acute coronary syndrome: a review. JAMA. 2021;325(15):1545-1555. doi:10.1001/jama.2021.0716
- Banerjee S, Angiolillo DJ, Boden WE, et al. Use of antiplatelet therapy/DAPT for Post-PCI patients undergoing noncardiac surgery. J Am Coll Cardiol. 2017;69(14):1861-1870. doi:10.1016/j.jacc.2017.02.012
- Schoos M, Chandrasekhar J, Baber U, et al. Causes, timing, and impact of dual antiplatelet therapy interruption for surgery (from the patterns of non-adherence to anti-platelet regimens in stented patients registry). Am J Cardiol. 2017;120(6):904-910. doi:10.1016/j.amjcard.2017.06.016
- Dangas G, Baber U, Sharma S, et al. Ticagrelor with or without aspirin after Complex PCI. J Am Coll Cardiol. 2020;75(19):2414-2424. doi:10.1016/j.jacc.2020.03.011
- Kim B, Hong S, Cho Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323(23):2407-2416. doi:10.1001/jama.2020.7580
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
- Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198(4):198-199. doi:10.5694/mja12.10614
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):160S-198S. doi:10.1378/chest.08-0670
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833. doi:10.1056/NEJMoa1501035
- Sunkara T, Ofori E, Zarubin V, Caughey ME, Gaduputi V, Reddy M. Perioperative management of direct oral anticoagulants (DOACs): a systemic review. Health Serv Insights. 2016;9(Suppl 1):25-36. doi:10.4137/HSI.S40701
- Noseworthy PA, Yao X, Shah ND, Gersh BJ. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease. Int J Cardiol. 2016;209:181-183. doi:10.1016/j.ijcard.2016.02.005
- Beaser AD, Cifu AS. Management of patients with atrial fibrillation. JAMA. 2019;321(11):1100-1101. doi:10.1001/jama.2019.1264
- López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. doi:10.1136/bmj.j5058
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76(5):594-622. doi:10.1016/j.jacc.2020.04.053
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179(11):1469-1478. doi:10.1001/jamainternmed.2019.2431
- Mujer MTP, Rai MP, Atti V, et al. An update on the reversal of non-vitamin K antagonist oral anticoagulants. Adv Hematol. 2020;2020:7636104. doi:10.1155/2020/7636104
- Santise G, Nardella S, Migliano F, Testa A, Maselli D. The HAS-BLED score is associated with major bleeding in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(6):1601-1606. doi:10.1053/j.jvca.2019.01.021
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736-2747. doi:10.1161/CIRCULATIONAHA.110.009449
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120(15):2954-2962. doi:10.1182/blood-2012-06-415943
- Oprea AD, Popescu WM. Perioperative management of antiplatelet therapy. Br J Anaesth. 2013;111 Suppl 1:3. doi:10.1093/bja/aet402
- Lakhanpal V, Schocket SS, Elman MJ, Dogra MR. Intraoperative massive suprachoroidal hemorrhage during pars plana vitrectomy. Ophthalmology. 1990;97(9):1114-1119. doi:10.1016/s0161-6420(90)32448-x
- Tabandeh H, Sullivan PM, Smahliuk P, Flynn HW, Schiffman J. Suprachoroidal hemorrhage during pars plana vitrectomy. risk factors and outcomes. Ophthalmology. 1999;106(2):236-242. doi:10.1016/S0161-6420(99)90062-3
- Kiernan DF, Hariprasad SM, Rusu IM, Mehta SV, Mieler WF, Jager RD. Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010;30(10):1573-1578. doi:10.1097/IAE.0b013e3181e2266d
- Kuhli-Hattenbach C, Fischer IB, Schalnus R, Hattenbach L. Subretinal hemorrhages associated with age-related macular degeneration in patients receiving anticoagulation or antiplatelet therapy. Am J Ophthalmol. 2010;149(2):316-321.e1. doi:10.1016/j.ajo.2009.08.033
- Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2013;33(3):621-626. doi:10.1097/IAE.0b013e3182671006
- Huebert I, Heinicke N, Kook D, et al. Dual platelet inhibition in cases of severe retrobulbar hemorrhage following retrobulbar and peribulbar anesthesia. J Cataract Refract Surg. 2015;41(10):2092-2101. doi:10.1016/j.jcrs.2015.10.051
- Herbert EN, Mokete B, Williamson TH, Laidlaw DaH. Haemorrhagic vitreoretinal complications associated with combined antiplatelet agents. Br J Ophthalmol. 2006;90(9):1209-1210. doi:10.1136/bjo.2006.095307
- Zarei M. Dual antiplatelet therapy may lead to hemorrhagic complications in vitrectomy of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):425-426. doi:10.1007/s00417-018-4151-1
- Shieh W, Sridhar J, Hong BK, et al. Ophthalmic complications associated with direct oral anticoagulant medications. Semin Ophthalmol. 2017;32(5):614-619. doi:10.3109/08820538.2016.1139738
- Jun JH, Hwang JC. Association of rivaroxaban anticoagulation and spontaneous vitreous hemorrhage. JAMA Ophthalmol. 2015;133(10):1184-1186. doi:10.1001/jamaophthalmol.2015.2069
- Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34 Suppl 12:S1-S18. doi:10.1097/IAE.0000000000000399
- Bemme S, Lauermann P, Striebe NA, et al. Risk of perioperative bleeding complications in rhegmatogenous retinal detachment surgery: a retrospective single-center study. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):961-969. doi:10.1007/s00417-019-04554-1
- Brillat E, Rouberol F, Palombi K, et al. A case-control study to assess aspirin as a risk factor of bleeding in rhegmatogenous retinal detachment surgery. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1899-1905. doi:10.1007/s00417-014-2900-3
- Passemard M, Koehrer P, Juniot A, Bron AM, Creuzot-Garcher C. Maintenance of anticoagulant and antiplatelet agents for patients undergoing peribulbar anesthesia and vitreoretinal surgery. Retina. 2012;32(9):1868-1873. doi:10.1097/IAE.0b013e31825097ae
- Fabinyi DCA, O'Neill EC, Connell PP, Clark JB. Vitreous cavity haemorrhage post-vitrectomy for diabetic eye disease: the effect of perioperative anticoagulation and antiplatelet agents. Clin Exp Ophthalmol. 2011;39(9):878-884. doi:10.1111/j.1442-9071.2011.02575.x
- Narendran N, Williamson TH. The effects of aspirin and warfarin therapy on haemorrhage in vitreoretinal surgery. Acta Ophthalmol Scand. 2003;81(1):38-40. doi:10.1034/j.1600-0420.2003.00020.x
- Kishore K, Mungee S. Comment on “risk of perioperative bleeding complications in rhegmatogenous retinal detachment surgery: a retrospective single-center study”: Bemme S et al. Graefes Arch Clin Exp Ophthalmol. 2020; 258(5). doi:10.1007/s00417-019-04554-1. Graefes Arch Clin Exp Ophthalmol. 2020;258(9):2071-2072. doi:10.1007/s00417-020-04723-7
- Lauermann P, Klingelhöfer A, Mielke D, et al. Risk factors for severe bleeding complications in vitreoretinal surgery and the role of antiplatelet or anticoagulant agents. Ophthalmol Retina. 2021;5(8):e23-e29. doi:10.1016/j.oret.2021.04.013
- Grand MG, Walia HS. Hemorrhagic risk of vitreoretinal surgery in patients maintained on novel oral anticoagulant therapy. Retina. 2016;36(2):299-304. doi:10.1097/IAE.0000000000000783
- Chandra A, Xing W, Kadhim MR, Williamson TH. Suprachoroidal hemorrhage in pars plana vitrectomy: risk factors and outcomes over 10 years. Ophthalmology. 2014;121(1):311-317. doi:10.1016/j.ophtha.2013.06.021
- Louison S, Gabrielle P, Soudry A, et al. Perioperative risk of bleeding with antithrombotic agents in macular surgery: a national, prospective, multicentre study. Acta Ophthalmol. 2020;98(8):e991-e997. doi:10.1111/aos.14434
- Meillon C, Gabrielle PH, Luu M, Aho-Glele LS, Bron AM, Creuzot-Garcher C. Antiplatelet and anticoagulant agents in vitreoretinal surgery: a prospective multicenter study involving 804 patients. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):461-467. doi:10.1007/s00417-017-3897-1
- Ryan A, Saad T, Kirwan C, Keegan DJ, Acheson RW. Maintenance of perioperative antiplatelet and anticoagulant therapy for vitreoretinal surgery. Clin Exp Ophthalmol. 2013;41(4):387-395. doi:10.1111/ceo.12017
- Khuthaila MK, Hsu J, Chiang A, et al. Postoperative vitreous hemorrhage after diabetic 23-gauge pars plana vitrectomy. Am J Ophthalmol. 2013;155(4):757-763. doi:10.1016/j.ajo.2012.11.004
- Malik AI, Foster RE, Correa ZM, Petersen MR, Miller DM, Riemann CD. Anatomical and visual results of transconjunctival sutureless vitrectomy using subconjunctival anesthesia performed on select patients taking anticoagulant and antiplatelet agents. Retina. 2012;32(5):905-911. doi:10.1097/IAE.0b013e31822f55c4
- Chandra A, Jazayeri F, Williamson TH. Warfarin in vitreoretinal surgery: a case controlled series. Br J Ophthalmol. 2011;95(7):976-978. doi:10.1136/bjo.2010.187526
- Brown JS, Mahmoud TH. Anticoagulation and clinically significant postoperative vitreous hemorrhage in diabetic vitrectomy. Retina. 2011;31(10):1983-1987. doi:10.1097/IAE.0b013e31821800cd
- Fu AD, McDonald HR, Williams DF, et al. Anticoagulation with warfarin in vitreoretinal surgery. Retina. 2007;27(3):290-295. doi:10.1097/01.iae.0000243033.39301.10
- Dayani PN, Grand MG. Maintenance of warfarin anticoagulation for patients undergoing vitreoretinal surgery. Trans Am Ophthalmol Soc. 2006;104:149-160.








