As the ever-expanding world of technology seeps more and more into clinical decision-making, the retinal physician can begin to feel overwhelmed by the constant input and output of data — the input of data into our own brains and the output from our brains into those of our patients. Digesting pertinent markers of pathology into well-defined clinical signs not only yields better understanding of disease but also creates trigger points in clinical decision-making. Optical coherence tomography (OCT), a mainstay in ophthalmic diagnostic testing for the modern retinal physician, lends itself especially well to the development of such signs. This article discusses some newer OCT signs that are not just descriptive of a pathologic process but also may trigger a change in your clinical judgment.
SHALLOW IRREGULAR RETINAL PIGMENT EPITHELIUM ELEVATION
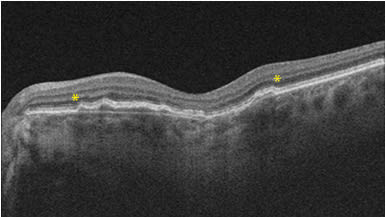
Shallow, irregular retinal pigment epithelium (RPE) elevation, or SIRE, stemmed from the double-layer sign initially described in polypoidal choroidal vasculopathy.1 When the RPE is elevated, as in type 1 macular neovascularization (MNV), the underlying hyperreflective Bruch’s membrane becomes visible,2 thus creating 2 hyperreflective lines or a double-layer sign. In patients with nonexudative age-related macular degeneration, presence of SIRE may predict that 1 in 4 of these patients have an underlying MNV that is not yet exudative.3 The features of SIRE include “a length of more than 1000 mm, RPE elevation predominantly less than 100 mm (resulting in shallow morphologic features), an irregular overlying RPE layer, and nonhomogenous reflectivity” (Figure 1).3 Histopathological studies from Project MACULA have confirmed presence of type 1 MNV within a SIRE, but caution that thick basal laminar deposits may present as a hyperreflective undulating line, and distinguishing this from a true SIRE may be important for assessing risk of conversion to exudative disease.4
INTERNAL LIMITING MEMBRANE DRAPE
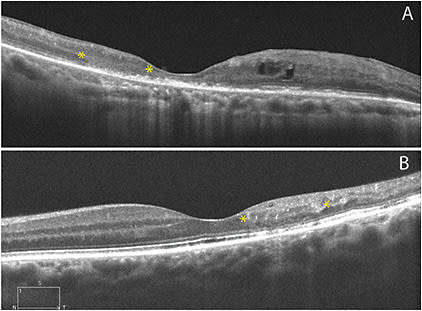
Macular telangiectasia (MacTel) type 2 is likely a neurodegenerative disorder primarily involving Mueller cells.5 The loss of tissue creates hyporeflective cavitations on OCT, which creates a draping effect of the internal limiting membrane (ILM) with preserved foveal contour (Figure 2).6 Importantly, the ILM drape sign may be masked by presence of subfoveal fluid from neovascularization.7 The ILM drape reappears as the acute fluid resolves.

DISORGANIZATION OF THE RETINAL INNER LAYERS
Accurately predicting visual outcome is an important aspect to crafting a treatment plan and managing patient expectation in various retinal vascular disorders. In diseases such as retinal vein occlusion and diabetic retinopathy, advanced stages of ischemia and edema can lead to disorganization of the retinal inner layers (DRIL) and is associated with visual acuity, especially when located within 1 mm of the fovea.8 Defined as the loss of distinguishable boundaries between the ganglion cell–inner plexiform layer complex, inner nuclear layer, and outer plexiform layer (Figure 3), the larger the horizontal extent of the DRIL, the poorer the visual prognosis.8 Perfusion abnormalities within the deep capillary plexus, leading to hypoxia, increased vascular endothelial growth factor production, and hence vascular permeability, appears to be associated with DRIL, and its presence is a risk factor for recurrent macular edema.9 Extrapolated to other macular diseases such as macular pucker, the presence of DRIL can alter the decision to treat or observe.10
BACILLARY LAYER DETACHMENT
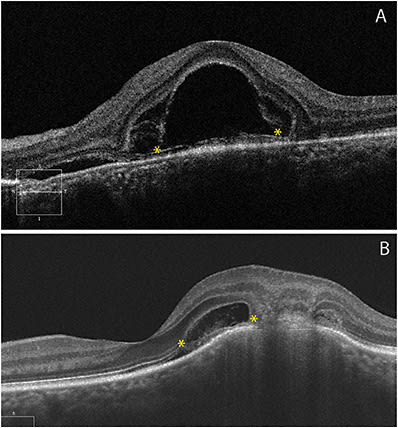
Fluid dissecting through the retina may result in an intraphotoreceptor fracture at the level of the myoids, constituting a bacillary layer detachment (BALAD) (Figure 4).11 BALAD has been observed in multiple retinal diseases including MNV,12 trauma,13 and inflammatory retinal disease.14,15 Synthesis of reported cases over time suggests that the main mechanism of BALAD may be similar to exudative retinal detachment.11 Furthermore, recognizing BALAD in neovascular age-related macular degeneration may predict the risk of subretinal fibrosis.12
PARACENTRAL ACUTE MIDDLE MACULOPATHY
Paracentral acute middle maculopathy (PAMM) arises as placoid, hyperreflective bands involving the inner plexiform layer, inner nuclear layer, and outer plexiform layer, sparing the outer retina (Figure 5).16 When viewed clinically, PAMM lesions are deeper with a smoother contour and more grayish coloration than a nerve fiber layer infarct (cotton wool spot) which may have a similar ischemic etiology. Near-infrared reflectance imaging reveals a hyporeflective but well-demarcated lesion corresponding to the area seen clinically and/or on spectral-domain OCT (SD-OCT).17 As the acute ischemia resolves, INL thinning persists.18
Paracentral acute middle maculopathy lesions can be isolated, associated with other retinal vascular occlusive disease, or a presenting sign for varied systemic disorders. Perivenular presence should prompt investigation for a retinal vein occlusion, and localized or diffuse lesions may indicate a branch or central retinal artery occlusion, respectively. Other etiologies include stimulant medications or oral contraceptives, migraines, severe hypovolemia, orbital compression injury, viral illness, or H1N1 influenza vaccination.17,19
ACUTE MACULAR NEUORETINOPATHY
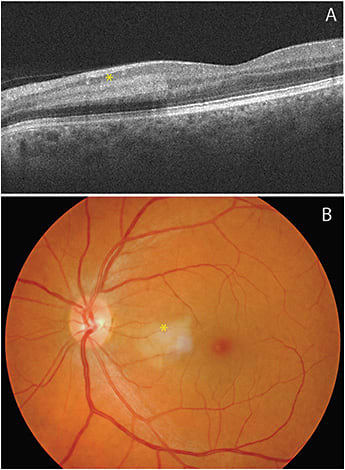
Acute macular neuroretinopathy (AMN) is a rare condition that also appears secondary to microvascular ischemia within the retina. Whereas PAMM is localized to the INL on SD-OCT, the hyperreflective band-like lesions in AMN affect the junction of the OPL and outer nuclear layer (Figure 6).20 The lesions may appear as dark, wedge-shaped, paracentral lesions but may be more apparent on near-infrared reflectance or red-free imaging as hyporeflective areas.21,22 Patients may have long-term visual sequelae from resultant atrophy of the ONL and ellipsoid zone. The microvascular origin of AMN is further illustrated by cases with coincident PAMM and AMN lesions; specifically, impairment in venous drainage may be a common link between these lesion types.18

CONCLUSION
While not all-inclusive, this list of pertinent OCT signs is key to deciding your next step when faced with many different disease entities. Recognizing SIRE may alert you to follow a patient more closely; persistent foveal DRIL may be the deciding factor to cease intravitreal anti-VEGF therapy; presence of BALAD may lead you to discuss realistic visual expectation in a patient with MNV. As the list of OCT signs lengthens with time, these entities will become as important as recognizing a salmon patch in sickle cell retinopathy, a keyhole pupil in ocular coloboma, or Dalen-Fuchs nodules in sympathetic ophthalmia. We use many clinical signs in our practices daily, and as our sophisticated technology entwines further into the clinical examination, these signs could lead to more accurate, timely diagnoses and better outcomes for patients. RP
REFERENCES
- Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007;27(5):589-594. doi:10.1097/01.iae.0000249386.63482.05
- Bloom SM, Singal IP. The outer Bruch membrane layer: a previously undescribed spectral-domain optical coherence tomography finding. Retina. 2011;31(2):316-323. doi:10.1097/IAE.0b013e3181ed8c9a
- Narita C, Wu Z, Rosenfeld PJ, et al. Structural OCT signs suggestive of subclinical nonexudative macular neovascularization in eyes with large drusen. Ophthalmology. 05 2020;127(5):637-647. doi:10.1016/j.ophtha.2019.11.007
- Sura AA, Chen L, Messinger JD, et al. Measuring the contributions of basal laminar deposit and Bruch’s membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61(13):19. doi:10.1167/iovs.61.13.19
- Gillies MC, Mehta H, Bird AC. Macular telangiectasia type 2 without clinically detectable vasculopathy. JAMA Ophthalmol. 2015;133(8):951-954. doi:10.1001/jamaophthalmol.2015.1796
- Paunescu LA, Ko TH, Duker JS, et al. Idiopathic juxtafoveal retinal telangiectasis: new findings by ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113(1):48-57. doi:10.1016/j.ophtha.2005.08.016
- Abdul-Rahman A. Case report: internal limiting membrane drape sign masking by foveal detachment in macular telangiectasia type 2. BMC Ophthalmol. 2020;20(1):213. doi:10.1186/s12886-020-01485-y
- Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309-1316. doi:10.1001/jamaophthalmol.2014.2350
- Costa JV, Moura-Coelho N, Abreu AC, Neves P, Ornelas M, Furtado MJ. Macular edema secondary to retinal vein occlusion in a real-life setting: a multicenter, nationwide, 3-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2021;259(2):343-350. doi:10.1007/s00417-020-04932-0
- Zur D, Iglicki M, Feldinger L, et al. Disorganization of retinal inner layers as a biomarker for idiopathic epiretinal membrane after macular surgery-the DREAM study. Am J Ophthalmol. 2018;196:129-135. doi:10.1016/j.ajo.2018.08.037
- Ramtohul P, Engelbert M, Malclès A, et al. Bacillary layer detachment: multimodal imaging and histologic evidence of a novel optical coherence tomography terminology: literature review and proposed theory. Retina. 2021;41(11):2193-2207. doi:10.1097/IAE.0000000000003217
- Ramtohul P, Malclès A, Gigon E, et al. Long-term outcomes of bacillary layer detachment in neovascular age-related macular degeneration. Ophthalmol Retina. 2022;6(3):185-195. doi:10.1016/j.oret.2021.09.010
- Tekin K, Teke MY. Bacillary layer detachment: a novel optical coherence tomography finding as part of blunt eye trauma. Clin Exp Optom. 2019;102(3):343-344. doi:10.1111/cxo.12876
- Fernández-Avellaneda P, Breazzano MP, Fragiotta S, et al. Bacillary layer detachment overlying reduced choriocapillaris flow in acute idiopathic maculopathy. Retin Cases Brief Rep. 2022;16(1):59-66. doi:10.1097/ICB.0000000000000943
- Agarwal A, Freund KB, Kumar A, et al. Bacillary layer detachment in acute Vogt-Koyanagi-Harada disease: a novel swept-source optical coherence tomography analysis. Retina. 2021;41(4):774-783. doi:10.1097/IAE.0000000000002914
- Sarraf D, Rahimy E, Fawzi AA, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131(10):1275-1287. doi:10.1001/jamaophthalmol.2013.4056
- Rahimy E, Kuehlewein L, Sadda SR, Sarraf D. Paracentral acute middle maculopathy: what we knew then and what we know now. Retina. 2015;35(10):1921-1930. doi:10.1097/IAE.0000000000000785
- Iovino C, Au A, Ramtohul P, et al. Coincident PAMM and AMN and insights into a common pathophysiology. Am J Ophthalmol. 2022;236:136-146. doi:10.1016/j.ajo.2021.07.004
- Chen X, Rahimy E, Sergott RC, et al. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160(1):26-34.e1. doi:10.1016/j.ajo.2015.04.004
- Hughes EH, Siow YC, Hunyor AP. Acute macular neuroretinopathy: anatomic localisation of the lesion with high-resolution OCT. Eye (Lond). 2009;23(11):2132-2134. doi:10.1038/eye.2008.430
- Bhavsar KV, Lin S, Rahimy E, et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538-565. doi:10.1016/j.survophthal.2016.03.003
- Fawzi AA, Pappuru RR, Sarraf D, et al. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina. 2012;32(8):1500-1513. doi:10.1097/IAE.0b013e318263d0c3








