Related
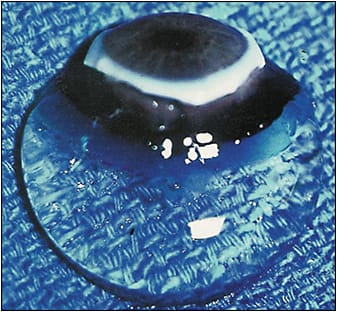
Vitreous is the largest structure within the eye, yet understanding of this enigmatic organ is perhaps less than any other in the eye.1-4 The role of vitreous in ocular physiology is poorly understood, but probably quite important,5 especially with respect to oxygen. An important aspect of how vitreous mitigates damaging effects of oxygen free radicals is the presence of antioxidants,6 especially ascorbate, with higher levels found in the posterior vitreous than centrally.7 This is presumably to protect the retina and optic nerve from harmful reactive oxygen species. One of the most important roles of vitreous is the maintenance of transparency (Figure 1),8,9 which is the focus of this article. To understand how vitreous clarity is compromised and what might be done to restore transparency, one must appreciate vitreous biochemistry and the structural changes that occur with aging, myopia, diabetes, and other disorders.
VITREOUS BIOCHEMISTRY AND MORPHOLOGY
Macromolecules comprise 2% of vitreous, and the remaining 98% is composed of water. Despite low concentrations, these macromolecules are responsible for the gel state of vitreous and its transparency. The 2 most important structural macromolecules are hyaluronan (HA), which provides viscoelasticity, and collagen (primarily type 2), which serves as the “skeleton” of the vitreous body. Type IX collagen is found on the surface of human collagen fibrils and may be very important in mediating the intricate supramolecular relation between HA and vitreous collagen, which promotes transparency by spreading collagen fibrils apart.10-12
FIBROUS LIQUEFACTION
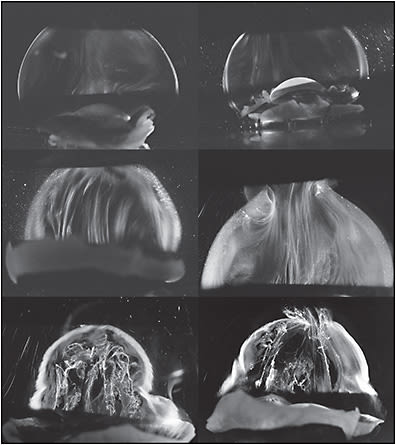
During aging, HA dissociates from collagen, resulting in simultaneous liquefaction, owing to the significant hydrophilicity of HA, and cross-linking of microscopic collagen fibrils (Figure 2) into macroscopic fibers that insert into the peripheral vitreous base and course anteroposteriorly (Figure 3). Dissociation of these macromolecules from their 3-dimensional supramolecular network destabilizes the vitreous body, ultimately resulting in its collapse and posterior vitreous detachment (PVD), which is innocuous if there is concomitant dehiscence at the vitreoretinal interface.

MYOPIC VITREOPATHY
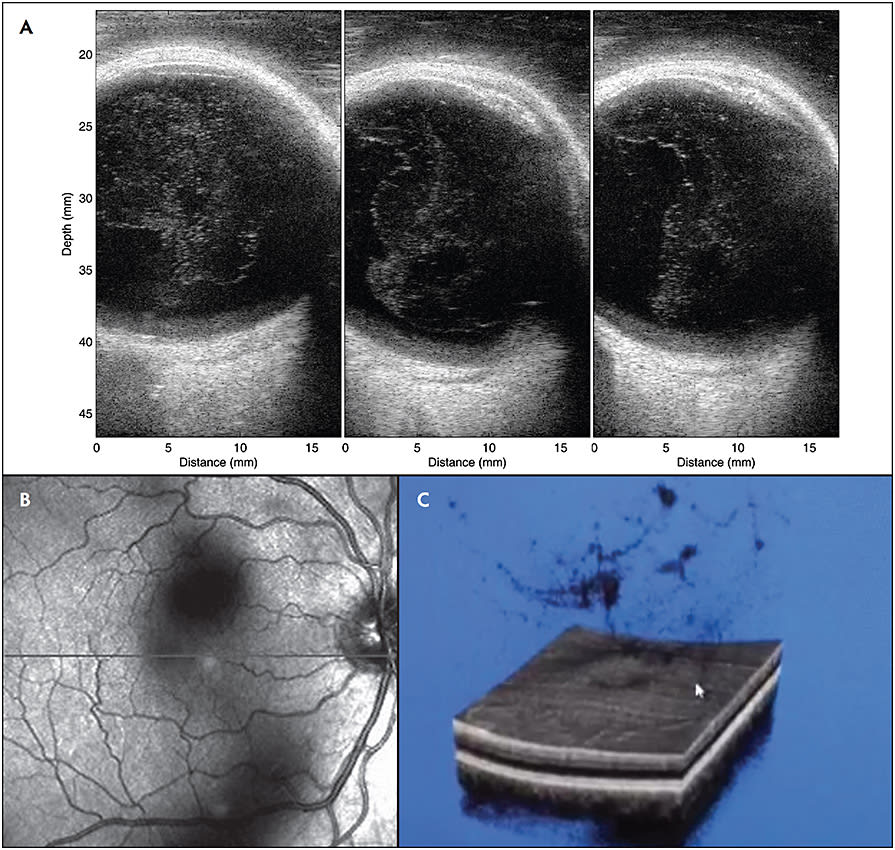
Fibrous liquefaction is accelerated in myopia, which is likely the consequence of both inherited and degenerative processes. The result is the appearance of collagen aggregates within the vitreous body (Figure 4A) and a loss of transparency at an early age (Figures 4B and 4C). Recent studies found greater vitreous density and correspondingly more degradation in contrast sensitivity function with increasing axial length.13 Myopic vitreopathy with fibrous liquefaction also contributes to collapse of the vitreous body early in life, at a time prior to weakening of vitreoretinal adhesion, thus resulting in anomalous PVD with various untoward effects.11
POSTERIOR VITREOUS DETACHMENT
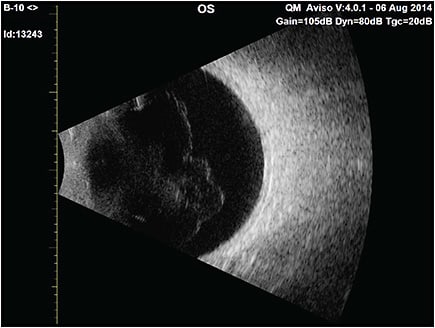
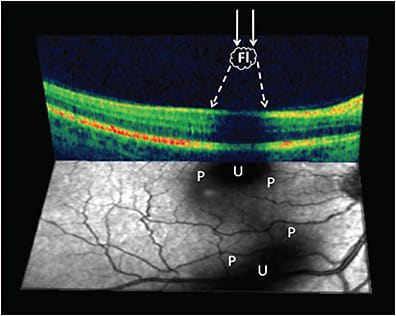
Posterior vitreous detachment (PVD)14 (Figure 5) occurs in two-thirds of people older than 65 years, but a decade or more earlier in myopes.15 In the absence of sufficient weakening of vitreoretinal adhesion, there can be untoward effects on vitreous (vitreoschisis16,17) and the retina, collectively known as anomalous PVD.18,19 Even in the absence of anomalous PVD, there can be untoward effects due to disturbance in transvitreal photon transmission to the retina, resulting in shadows (Figure 4B) and the visual phenomenon of floaters that can at times be extremely bothersome. The combined effects of central vitreous opacities and the dense collagen matrix of the posterior vitreous cortex along with folding of the detached posterior vitreous11 appear to severely impact the passage of light to the retina and disturb vision. Such cases suffer from vision degrading myodesopsia (VDM), a diagnosis based on objective quantitative criteria.
VISION DEGRADING MYODESOPSIA
The most common consequence of PVD is the sudden onset of the visual phenomenon of floaters. The literature and common vernacular unfortunately use this term to refer to opacities within the vitreous body that induce the visual phenomena, thus greater linguistic precision is desirable. Vitreous floaters are not structures within the vitreous body, but entopic images that disturb vision. In the absence of uveitis or vitreous hemorrhage, it is not clear why some individuals with vitreous opacities are extremely bothered by vitreous floaters, while others are not. Measurable increased density of vitreous structure and concomitant degradation in vision form the basis for diagnosing VDM. The term “myodesopsia” is derived from the Greek myiodes, meaning flylike, and opsis, meaning vision.11
The way that VDM disturbs vision is by degrading contrast sensitivity function (CSF),20 which negatively affects psychosocial well-being.21-24 The negative impact of floaters on health-related quality of life has been shown to be comparable to age-related macular degeneration, diabetic retinopathy, and myopia, as well as systemic diseases such as mild angina, colon cancer, and asymptomatic HIV infection.21 Although most patients with floaters do not have significant diminution of visual acuity, CSF is degraded by an average of 91.3% in patients with VDM compared to age-matched controls.25 Similarly, in patients with myopic vitreopathy, CSF was shown to be reduced compared with controls, with pronounced degradation in patients with both myopic vitreopathy centrally and PVD posteriorly.13
From a molecular standpoint, the exact mechanisms for these disturbances in vision are not currently known, but recent investigations are beginning to shed some light. Preliminary studies have shown that vitreous excised from patients with VDM had a two-fold increase in particle numbers and doubling of particle sizes compared with controls.26 Huang et al27 postulated that tortuous aggregates of vitreous fibers with nodularities within the vitreous body in conjunction with the detached posterior vitreous cortex and its dense matrix of collagen fibrils would scatter light in a “disturbing” manner, causing vision degradation due to the optical effects of the penumbra cast by the irregular structures of the opacified vitreous (Figure 6).
DIAGNOSTIC IMAGING
Although physical examination can image prominent vitreous opacities, there are limitations to the illumination-observation angle with slit-lamp biomicroscopy and no Tyndall effect with ophthalmoscopy. Ultrasonography is currently the best way to evaluate patients who complain of vitreous floaters, primarily because it enables evaluation of the entire vitreous body (Figure 5), not just the posterior aspects imaged by optical coherence tomography (OCT). Quantitative ultrasonography (QUS) was developed to provide objective assessment of vitreous density with a probe optimized for vitreous imaging.28 A clinical study showed that QUS measured greater vitreous density in subjects with PVD compared to controls without PVD, and that there is increasing vitreous echodensity and decreased CSF in the PVD group with increasing age,29 suggesting that not only do eyes with PVD have greater vitreous opacification than eyes without PVD, but that after PVD occurs, there is progressive aggregation of vitreous collagen fibrils in the vitreous body and/or increased folding of the detached posterior vitreous cortex, further degrading vision. Although imaging with sound is practical and useful, imaging with light would more closely approximate the phenomenon of VDM (Figure 4B and Figure 6). It is anticipated that technologies that measure light scattering26,30 in vivo will elucidate the mechanism(s) by which vitreous causes VDM. Ultimately, the goal is to provide therapies that reduce, eliminate, and perhaps one day prevent the deleterious impact of VDM on vision and quality of life. RP
Editor’s note: Parts 2 and 3 of this series will be available with subsequent issues at www.retinalphysician.com .
References
- Sebag J. Classifying posterior vitreous detachment: a new way to look at the invisible. Br J Ophthalmol. 1997;81(7):521. doi:10.1136/bjo.81.7.521
- Sebag J. Seeing the invisible: the challenge of imaging vitreous. J Biomed Opt. 2004;9(1):38. doi:10.1117/1.1627339
- Sebag J. To see the invisible: the quest of imaging vitreous. Dev Ophthalmol. 2008;42:5-28. doi:10.1159/000138754
- Sebag J. Vitreous: the resplendent enigma. Br J Ophthalmol. 2009;93(8):989-991. doi:10.1136/bjo.2009.157313
- Chew L, Sebag J. Vitreous. In: Adler’s Physiology of the Eye. 12th ed. Elsevier; 2022 (in press).
- Ankamah E, Sebag J, Ng E, Nolan JM. Vitreous antioxidants, degeneration, and vitreo-retinopathy: exploring the links. Antioxidants. 2020;9(1):1-20. doi:10.3390/antiox9010007
- Murali K, Kang D, Nazari H, et al. Spatial variations in vitreous oxygen consumption. PLoS One. 2016;11(3):1-13. doi:10.1371/journal.pone.0149961
- Sebag J. The Vitreous: Structure, Function, and Pathobiology. Springer-Verlag; 1989.
- Sebag J, ed. Vitreous in Health and Disease. Springer; 2014.
- Bishop P. The biochemical structure of mammalian vitreous. Eye. 1996;10(6):664-670. doi:10.1038/eye.1996.159
- Sebag J. Vitreous and vision degrading myodesopsia. Prog Retin Eye Res. 2020;79:100847. doi:10.1016/j.preteyeres.2020.100847
- Crafoord S, Ghosh F, Sebag J. Vitreous biochemistry and artificial vitreous. In: Sebag J, ed. Vitreous in Health and Disease. Springer; 2014:81-94.
- Nguyen JH, Nguyen-Cuu J, Mamou J, Routledge B, Yee KMP, Sebag J. Vitreous structure and visual function in myopic vitreopathy causing vision degrading myodesopsia. Am J Ophthalmol. 2021;224:246-253. doi:10.1016/j.ajo.2020.09.017
- Sebag J. Posterior vitreous detachment. Ophthalmology. 2018;125(9):1384-1385. doi:10.1016/j.ophtha.2018.05.018
- Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100(9):1384-1388. doi:10.1016/S0161-6420(93)31471-5
- Sebag J. Vitreoschisis. Graefe’s Arch Clin Exp Ophthalmol. 2008;246(3):329-332. doi:10.1007/s00417-007-0743-x
- Sebag J, Niemeyer M, Koss MJ. Anomalous PVD and vitreoschisis. In: Sebag J, ed. Vitreous in Health and Disease. Springer; 2014:241-265.
- Sebag J. Anomalous PVD — a unifying concept in vitreo-retinal diseases. Graefe’s Arch Clin Exp Ophthalmol. 2004;242(8):690-698. doi: 10.1007/s00417-004-0980-1.
- Sebag J. Vitreous anatomy, aging, and anomalous posterior vitreous detachment. In: Dartt DA, Besharse JC, Dana R, eds. Encyclopedia of the Eye. Elsevier; 2010:307-315.
- Garcia GA, Khoshnevis M, Yee KMP, Nguyen-Cuu J, Nguyen JH, Sebag J. Degradation of contrast sensitivity function following posterior vitreous detachment. Am J Ophthalmol. 2016;172:7-12. doi:10.1016/j.ajo.2016.09.005
- Wagle AM, Lim WY, Yap TP, Neelam K, Au Eong KG. Utility values associated with vitreous floaters. Am J Ophthalmol. 2011;152(1):60-65.e1. doi:10.1016/j.ajo.2011.01.026
- Sebag J. Floaters and the quality of life. Am J Ophthalmol. 2011;152(1):3-4.e1. doi:10.1016/j.ajo.2011.02.015
- Cipolletta S, Beccarello A, Galan A. A psychological perspective of eye floaters. Qual Health Res. 2012;22(11):1547-1558. doi:10.1177/1049732312456604
- Kim YK, Moon SY, Yim KM, Seong SJ, Hwang JY, Park SP. Psychological distress in patients with symptomatic vitreous floaters. J Ophthalmol. 2017;2017:3191576. doi:10.1155/2017/3191576.
- Sebag J, Yee KMP, Nguyen JH, Nguyen-Cuu J. Long-term safety and efficacy of limited vitrectomy for vision degrading myodesopsia resulting from vitreous floaters. Ophthalmol Retina. 2018;2(9):881-887. doi:10.1016/j.oret.2018.03.011
- Kershaw K, Nguyen D, Yee K, Nguyen J, Harrington M, Sebag J. Dynamic light scattering of vitreous in patients with vitreous floaters compared to macular pucker. Invest Ophthalmol Vis Sci. 2017;58(8):4829.
- Huang L, Yee K, Wa C, Nguyen J, Sadun A, Sebag J. Vitreous floaters and vision: current concepts and management paradigms. In: Sebag J, ed. Vitreous — in Health and Disease. Springer; 2014:771-788.
- Mamou J, Wa CA, Yee KMP, Silverman RH, Ketterling JA, Sadun AA, Sebag J. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Invest Ophthalmol Vis Sci. 2015;56(3):1611-1617. doi:10.1167/iovs.14-15414
- Garcia G, Khoshnevis M, Nguyen-Cuu J, et al. The effects of aging vitreous on contrast sensitivity function. Graefe’s Arch Clin Exp Ophthalmol. 2018;256(5):919-925. doi:10.1007/s00417-018-3957-1
- Mura M, Engelbrecht LA, de Smet MD, Papadaki TG, van den Berg TJ, Tan HS. Surgery for floaters. Ophthalmology. 2011;118(9):1894e1894.e1. doi:10.1016/j.ophtha.2011.05.020.








