Diabetic retinopathy (DR) is a major cause of preventable blindness worldwide, affecting 4.1 million patients in the United States. Approximately 17% of pregnancies are impacted by DR-associated complications, making DR a leading physiologic complication of pregnancy, with potential for lasting visual ramifications.1,2 Patients managing their diabetic comorbidities with insulin are at higher risk; up to 27% of these patients also develop DR over the course of their pregnancy.2-4 Because DR progresses twice as fast in pregnancy, this subset of patients is particularly vulnerable to vison-threatening pathology.
This narrative review summarizes the underlying mechanisms of pregnancy in development and exacerbation of DR, and key risk factors associated with development of DR in pregnancy. Additionally, it will examine current evidence-based screening guidelines and promising future directions in this field.
MECHANISMS OF DR IN PREGNANCY
Numerous mechanisms have been proposed to explain the effect of pregnancy in development and progression of DR. Broadly, these include physiologic pressures unique to the state of pregnancy, hormonal alterations, and upregulation of angiogenesis.
Physiologic Pressures
Pregnancy is characterized by increases in cardiac output, total peripheral resistance, and total circulatory volume. These changes are mediated by modification of the renin-angiotensin-aldosterone system and the hypothalamus-pituitary-adrenal axis. Generally, these alterations in systemic vasculature significantly increase pulse pressure, resulting in a state of hyperdynamic circulation in which shearing forces are intensified in the endothelium of capillary beds. In retinal microvasculature, these forces have been shown to decrease retinal pericyte count and result in a dysfunctional endothelial barrier. The retinal circulation is at even higher risk in a diabetic state, in which endothelial function is already compromised due to longstanding periods of hyperglycemia.5,6
Proangiogenic State
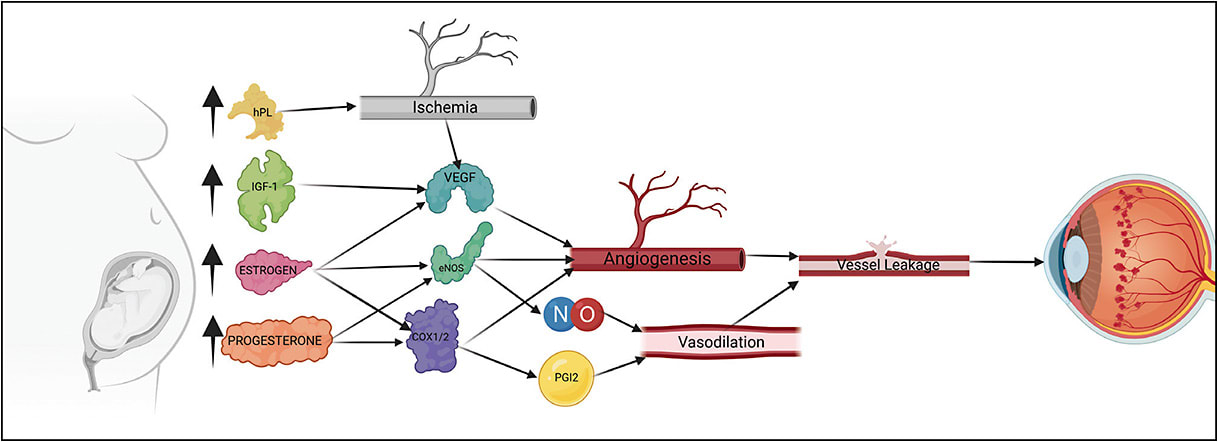
Pregnancy may be seen as a proangiogenic state because blood vessel growth is essential to meet metabolic demands of both the fetus and the mother. This angiogenic state is mediated by vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1), both of which have been shown to be markedly increased during pregnancy. In retinal tissue that is compromised by blood-retinal barrier breakdown from DR, aberrant increases in circulating VEGF have potential to generate neovascularization.5 Increases in neovascularization in turn promote vessel growth and permeability, resulting in pathologic retinal edema. Studies examining IGF-1 in pregnancy have shown mostly correlative results. In 2003, Loukovaara et al found serum IGF-1 levels in pregnant patients to be associated with worsening of DR.7,8 Lauszus et al also examined serum levels of IGF-1 in pregnant patients, finding patients whose DR progressed during pregnancy had significantly higher IGF-1 levels than those who experienced no progression.9 Interestingly, while insulin and IGF-1 can bind to similar receptor domains, increased levels of insulin lispro as well as endogenous insulin have not been shown to worsen DR status. Potentially refuting this hypothesis, in 2005, Loukovaara et al found systemic levels of IGF-1 and IGFB3 not to correlate with progression of DR.10-12 These changes may have to do with the integrity of the blood-retinal barrier, which can be compromised to varying extents in these patients. Fluorescein angiographic analysis would be beneficial in future studies to further test this hypothesis.
Human Placental Lactogen, COX-1, and eNOS
Altered hormonal signals and gene expression also drive a proangiogenic state, accelerating the progression of DR. Specifically, COX, eNOS gene, and human placental lactogen (hPL) are all implicated to some degree; however, a single mechanism has not been completely elucidated. During pregnancy, increases in estrogen, progesterone-derived hormones, and hPL have been shown to induce vascular changes leading to DR.13 Estrogen and progesterone are specifically known to have significant impacts on vascular function and are most prominent in the uterine arteries responsible for vascular supply to the growing fetus.14 Rising progesterone levels throughout pregnancy are also responsible for increases in vasodilator production via the same genes targeted by estrogen (eNOS, COX-1, NO, PGI2).13,14 The drastic changes in vasculature caused by estrogen and progesterone impact retinal vasculature as well, causing mass angiogenesis and pathologic retinal edema, placing further strain on retinal tissue. Because hPL is produced in large quantities during pregnancy, Mallika et al cited it as one of the major contributors to the progression of DR in pregnancy.6
RISK FACTORS FOR PROGRESSION OF DR DURING PREGNANCY
A multitude of studies have aimed to explore the association between pregnancy and the progression of DR. As described above, the presence of a proangiogenic state during pregnancy creates a systemic environment that is, itself, a risk factor for progression of DR.
Pregnancy as a Risk Factor
Current literature appears to suggest that the first and second trimesters incur heightened risk for progression of DR, whereas the third trimester may provide a reduction in risk. Bourry et al studied 359 patients with 499 pregnancies examining progression by trimester, defining progression as an increase in one or more of the Early Treatment in Diabetic Retinopathy Study stages of classification of DR. It was found that 11.0% of progressions occurred in the first trimester, 9.2% within the second trimester, and 3.8% within the third trimester.15 Egan et al followed 185 women with pregestational diabetes and singleton pregnancies, defining progression as at least one stage of deterioration of DR and/or the presence of DME in at least 1 eye. Forty-eight (25.9%) of subjects progressed, compared to 134 (74.1%) who did not progress.16 In a 2007 study by Loukovaara et al, progression was determined if the DCCT score of a patient increased by one or more levels during pregnancy. They recorded a statistically significant difference between the number of women who progressed in their stage of DR and the number of women who did not in the sample of pregnant women (62% and 38%, respectively, with P=.005). It has been hypothesized that progression in the first trimester is likely a result of the rapid hormonal changes and adaptations that occur in that time.17
Systemic Risk Factors
Numerous systemic risk factors have been identified to be associated with progression of DR during the gestational period that coincide with systemic manifestations of diabetic status (Table 1). Concomitant diabetic nephropathy and neuropathy have been shown through a multitude of studies to be associated with worsening DR status. However, conflicting evidence is present regarding whether hypertension is a significant predictor of DR progression in pregnant women.7,12,15,18-20 Egan et al found convincing evidence that increased systolic and diastolic blood pressure confers an increased risk of progression of DR (OR=1.03, P=.02) and Makwana et al found a significant difference between mean diastolic blood pressure between pregnant women with and without DR (P=.0017). However, many studies conflict with these results.8,12,16,18,21 Rasmussen et al, Loukovaara et al, and Bourry et al found no association between hypertension and increased risk of DR progression, making this relationship an arena for investigation across larger patient cohorts.7,10,12,15,18,20
| RISK FACTOR | STUDY | SAMPLE SIZE | STATISTICAL SIGNIFICANCE |
| Trimester of pregnancy | Bourry15 | 375 patients, 499 pregnancies | First trimester: 11% progressedSecond trimester: 9.2% progressed Third trimester: 3.8% progressed |
| Egan16 | 185 pregnant women | P=.01 between those who progressed and those who did not in the first trimester P=.89 between those who progressed and those who did not in the third trimester |
|
| Loukovaara7 | 54 pregnant women (45 with diabetes, 9 control) | P=.005 between the number of women who progressed and those who did not between pregnant and nonpregnant groups | |
| Systemic risk factor: hypertension | Bourry15 | 375 patients, 499 pregnancies | P=.95 between those who progressed and those who did not progress |
| Klein19 | 374 women (gradable images from 133 pregnant and 241 nonpregnant women) | P=.105 between pregnant and nonpregnant women’s systolic blood pressure P<.001 between pregnant and nonpregnant women’s diastolic blood pressure |
|
| Rasmussen20 | 80 pregnant women | P=.5 between pregnant women who progressed and did not progress for systolic blood pressure P=.18 between pregnant women who progressed and did not progress for diastolic blood pressure |
|
| Loukovaara7 | 54 pregnant women (45 with diabetes, 9 control) | P=.576 between pregnant and nonpregnant women | |
| Egan16 | 185 pregnant women | P=.02 increased odds of progression due to increased booking systolic blood pressure in pregnant women P=.03 between those who progressed and those who did not progress |
|
| Makwana21 | 50 pregnant women | P=.0017 for mean diastolic pressure between pregnant women with and without diabetic retinopathy (DR) | |
| Coexisting diabetic complications: nephropathy/proteinuria/neuropathy | Larinkari23 | 57 pregnant women | 0% of those with minimal DR and 53.3% of those with frank DR progressed |
| Cassar24 | 15 patients, 92 pregnancies | 22.2% of those with minimal DR at baseline developed nephropathy | |
| Klein19 | 374 women (gradable images from 133 pregnant and 241 nonpregnant women) | P=.644 between those who are pregnant and nonpregnant | |
| Makwana21 | 50 pregnant women | No proteinuria present in any DR or non-DR patients | |
| Bourry15 | 375 patients, 499 pregnancies | 2.2% with neuropathy, 7.6% with nephropathy | |
| Loukovaara7 | 54 pregnant women (45 with diabetes, 9 control) | P=.576 between diabetic and nondiabetic pregnant women | |
| Coexisting ocular disease | Klein19 | 374 women (gradable images from 133 pregnant and 241 nonpregnant women) | P=.574 between pregnant and nonpregnant women for the instance of central retinal vein occlusion P=.351 between pregnant and nonpregnant women for the instance of central retinal artery occlusion |
Coexisting Diabetic Complications
Recent studies have arrived at the conclusion that nephropathy does not significantly correlate with progression of DR in pregnant women. Makwana et al and Loukovaara et al noted that proteinuria/nephropathy was not present in any patients regardless of DR status.10-12,21 Bourry et al concluded that, contrary to previous studies, patients who have nephropathy do not seem to be at risk for progression of DR.15
Although diabetic neuropathy presented in 11 out of 499 of the subjects in the Bourry et al study (2.2%), the general clinical value of diabetic neuropathy in pregnant patients with DR is not well established. Because microvascular compromise would be expected to manifest with a degree of neuropathy concomitant with multiple other findings, the predictive value of diabetic neuropathy in pregnant patients with DR remains to be established.
Coexisting Ocular Diseases
In addition to the presence of systemic diabetic manifestations, the presence of ocular comorbidities also increases the likelihood of DR development or progression, leading to worsening visual outcomes for at-risk patients. The presence of central retinal artery occlusion (CRAO) and central retinal vein occlusion (CRVO) has been associated with worsening of diabetic status in pregnant women. Because of increased levels of estrogen during pregnancy, patients may be at increased risk of retinal artery and vein occlusions. However, in current literature, there has been no statistically significant difference between pregnant and nonpregnant women and the incidence of CRVO (P=.574) or CRAO, (P=.351).19
Although there are many pregnancy-related factors that are known to increase the chance of progression of DR in pregnant women, it seems that most of the expected risk factors that this narrative review analyzed were not consistent predictors across various studies. Larger sample sizes and the incorporation of a control group could provide more information on the specific relationship between progression of DR and controversial risk factors, such as hypertension and coexisting ocular disease. This could also help determine the degree of impact these risk factors can have on the progression of DR throughout pregnancy.
Diabetic Retinopathy Evaluation Recommendations
Current guidelines from the American Academy of Ophthalmology strongly advise that all pregnant diabetic women, regardless of ophthalmic status, should have an ophthalmologic examination before conception to determine the baseline severity of individual disease (Table 4).22 The obstetrician or primary care physician’s referral practices also play a crucial role in management and screening precautions. Additional visits are recommended during the first trimester and every 1 to 3 months for severe cases of DR until delivery to evaluate for disease presence.22 Based on the above information provided by Egan et al and others, future guidelines may need to consider if HTN and CRAO patients might qualify for further screenings.
| TYPE OF DIABETES/DIABETIC RETINOPATHY | RECOMMENDED INITIAL EVALUATION | RECOMMENDED FOLLOW-UP |
| No diabetic retinopathy to mild or moderate nonproliferative diabetic retinopathy | Soon after conception and early in the first trimester | Every 3-12 months |
| Severe nonproliferative diabetic retinopathy or worse | Soon after conception and early in the first trimester | Every 1-3 months |
| Gestational diabetes | Not required | Not required |
| *Adapted from Table 2 of the Diabetic Retinopathy Preferred Practice Pattern 2022 | ||
Pregnancy-related Conditions With Retinal Implications
In addition to systemic and ocular risk factors, physiologic consequences of pregnancy can also increase risk of DR development and progression (Tables 2 and 3). Gestational hypertension, defined as new-onset hypertension after 20 weeks of gestation, has been shown by Bourry et al to be associated with increased risk of development of DR.
| RISK FACTOR | STUDY | SAMPLE SIZE | STATISTICAL SIGNIFICANCE |
| Preeclampsia | Loukovaara8 | 52 pregnant women (45 with diabetes, 7 control) | P=.188 between diabetic and control pregnant women for c-reactive protein levels throughout pregnancyP=.216 between diabetic and control pregnant women for interleukin-6 levels throughout pregnancy P=.682 between diabetic and control pregnant women for vascular cell adhesion molecule-1 levels throughout pregnancy |
| Egan16 | 185 pregnant women | P=.80 between those who progressed and those who did not of those who had preeclampsia | |
| Rasmussen20 | 80 pregnant women | P=.80 between those who progressed and those who did not of those who had preeclampsia | |
| Gestational hypertension | Feghali25 | 192 pregnant women (60 with elective Caesarean section [c-section] delivery, 69 with c-section delivery prior to second stage of labor, 63 with vaginal or c-section delivery in the second stage) | 20% of elective c-section 29% of c-section delivery prior to second labor stage 22% of vaginal or c-section delivery in the second labor stage |
| RISK FACTOR | STUDY | SAMPLE SIZE | STATISTICAL SIGNIFICANCE |
| Duration of pregnancy | Loukovaara8 | 52 pregnant women (45 with diabetes, 7 control) | P<.0001 between pregnant women with and without diabetes |
| Gravida | Egan16 | 185 pregnant women | P=.17 between the gravida of those who progressed and those who did not |
| Parity | Egan16 | 185 pregnant women | P=.09 between the parity of those who progressed and those who did not |
If pregnancy is further complicated by preeclampsia, defined as the presence of gestational hypertension with the presence of end-organ damage, DR, in theory, would have even greater potential to significantly worsen. However, clinical studies have not shown such an association. Loukovaara et al found no significant impact of presence of preeclampsia on progression of DR.10-12 Egan et al also found no correlation between the DR progression of those with preeclampsia and those without (P=.8).16 Rasmussen et al furthermore found no significant correlation between mean number of patients with preeclampsia who progressed and mean number of patients with preeclampsia who did not progress (1 and 4, respectively, P=.68).20
CONCLUSION
There are various factors that contribute to the progression of DR. Primarily, pregnancy itself has been established to be an agonist for the progression of DR. Physiologic pressures and expression of genes such as hPL, eNOS, and COX are responsible for vasculature changes that are associated with pregnancy, increasing the risk of retinal ischemia or rupture due to angiogenesis. Most findings concur that the highest risk for progression occurs during the first trimester of pregnancy, whereas the third trimester provides a reduced risk. Pregnancy-related complications may also worsen the progression of DR. Further research in this area will be essential in updating current screening guidelines so physicians may best identify high-risk patients as well as trimesters in which these patients are at the highest risk for progression of DR. RP
REFERENCES
- Best RM, Chakravarthy U. Diabetic retinopathy in pregnancy. Br J Ophthalmol. 1997;81(3):249-251. doi:10.1136/bjo.81.3.249
- Morrison JL, Hodgson LA, Lim LL, Al-Qureshi S. Diabetic retinopathy in pregnancy: a review: Diabetic retinopathy in pregnancy. Clin Experiment Ophthalmol. 2016;44(4):321-334. doi:10.1111/ceo.12760
- Hadden DR. Diabetes in pregnancy 1985. Diabetologia. 1986;29(1):1-9. doi:10.1007/BF02427272
- Chan WC, Lim LT, Quinn MJ, Knox FA, McCance D, Best RM. Management and outcome of sight-threatening diabetic retinopathy in pregnancy. Eye. 2004;18(8):826-832. doi:10.1038/sj.eye.6701340
- Mrugacz M, Bryl A, Zorena K. Retinal vascular endothelial cell dysfunction and neuroretinal degeneration in diabetic patients. J Clin Med. 2021;10(3):458. doi:10.3390/jcm10030458
- Mallika P, Tan A, Aziz S, Asok T, Alwi SS, Intan G. Diabetic retinopathy and the effect of pregnancy. Malays Fam Physician. 2010;5(1):2-5.
- Loukovaara S, Immonen I, Teramo KA, Kaaja R. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care. 2003;26(4):1193-1198. doi:10.2337/diacare.26.4.1193
- Loukovaara S, Harju M, Kaaja R, Immonen I. Retinal capillary blood flow in diabetic and nondiabetic women during pregnancy and postpartum period. Invest Ophthalmol Vis Sci. 2003;44(4):1486-1491. doi:10.1167/iovs.02-0293
- Lauszus F, Klebe JG, Bek T. Diabetic retinopathy in pregnancy during tight metabolic control. Acta Obstet Gynecol Scand. 2000;79(5):367-370.
- Loukovaara S, Immonen IJ, Yandle TG, Nicholls G, Hiilesmaa VK, Kaaja RJ. Vasoactive mediators and retinopathy during type 1 diabetic pregnancy. Acta Ophthalmol Scand. 2005;83(1):57-62. doi:10.1111/j.1600-0420.2005.00384.x
- Loukovaara S, Immonen IJ, Koistinen R, et al. The insulin-like growth factor system and Type 1 diabetic retinopathy during pregnancy. J Diabetes Complicat. 2005;19(5):297-304. doi:10.1016/j.jdiacomp.2005.03.004
- Loukovaara S, Immonen I, Koistinen R, Hiilesmaa V, Kaaja R. Inflammatory markers and retinopathy in pregnancies complicated with type I diabetes. Eye (Lond). 2005;19(4):422-430. doi:10.1038/sj.eye.6701499
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1):R27-R44. doi:10.1530/JOE-16-0340
- Sennlaub F, Valamanesh F, Vazquez-Tello A, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108(2):198-204. doi:10.1161/01.CIR.0000080735.93327.00
- Bourry J, Courteville H, Ramdane N, et al. Progression of diabetic retinopathy and predictors of its development and progression during pregnancy in patients with type 1 diabetes: a report of 499 pregnancies. Diabetes Care. 2021;44(1):181-187. doi:10.2337/dc20-0904
- Egan AM, McVicker L, Heerey A, Carmody L, Harney F, Dunne FP. Diabetic retinopathy in pregnancy: a population-based study of women with pregestational diabetes. J Diabetes Res. 2015;2015:310239. doi:10.1155/2015/310239
- Kumar P, Magon N. Hormones in pregnancy. Niger Med J. 2012;53(4):179-183. doi:10.4103/0300-1652.107549
- Loukovaara S, Immonen IR, Loukovaara MJ, Koistinen R, Kaaja RJ. Glycodelin: a novel serum anti-inflammatory marker in type 1 diabetic retinopathy during pregnancy. Acta Ophthalmol Scand. 2007;85(1):46-49. doi:10.1111/j.1600-0420.2006.00766.x
- Klein BE, Klein R. Gravidity and diabetic retinopathy. Am J Epidemiol. 1984;119(4):564-569. doi:10.1093/oxfordjournals.aje.a113773
- Rasmussen KL, Laugesen CS, Ringholm L, Vestgaard M, Damm P, Mathiesen ER. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53(6):1076-1083. doi:10.1007/s00125-010-1697-9
- Makwana T, Takkar B, Venkatesh P, et al. Prevalence, progression, and outcomes of diabetic retinopathy during pregnancy in Indian scenario. Indian J Ophthalmol. 2018;66(4):541-546. doi:10.4103/ijo.IJO_1062_17
- Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic Retinopathy Preferred Practice Pattern. Ophthalmology. 2020;127(1):P66-P145. doi:10.1016/j.ophtha.2019.09.025
- Larinkari J, Laatikainen L, Ranta T, Mörönen P, Pesonen K, Laatikainen T. Metabolic control and serum hormone levels in relation to retinopathy in diabetic pregnancy. Diabetologia. 1982;22(5):327-332. doi:10.1007/BF00253576
- Cassar J, Kohner EM, Hamilton AM, Gordon H, Joplin GF. Diabetic retinopathy and pregnancy. Diabetologia. 1978;15(2):105-111. doi:10.1007/BF00422254
- Feghali M, Miodovnik M. Diabetes: hypertension during pregnancy and future diabetes mellitus. Nat Rev Endocrinol. 2013;9(8):446-447. doi:10.1038/nrendo.2013.110








